Abstract
BACE1 initiates the generation of the β-amyloid peptide, which likely causes Alzheimer’s disease (AD) when accumulated abnormally. BACE1 inhibitory drugs are currently being developed to treat AD patients. To mimic BACE1 inhibition in adults, we generated BACE1 conditional knockout (BACE1fl/fl) mice and bred BACE1fl/fl mice with ubiquitin-CreER mice to induce deletion of BACE1 after passing early developmental stages. Strikingly, sequential and increased deletion of BACE1 in an adult AD mouse model (5xFAD) was capable of completely reversing amyloid deposition. This reversal in amyloid deposition also resulted in significant improvement in gliosis and neuritic dystrophy. Moreover, synaptic functions, as determined by long-term potentiation and contextual fear conditioning experiments, were significantly improved, correlating with the reversal of amyloid plaques. Our results demonstrate that sustained and increasing BACE1 inhibition in adults can reverse amyloid deposition in an AD mouse model, and this observation will help to provide guidance for the proper use of BACE1 inhibitors in human patients.
Introduction
Alzheimer’s disease (AD), which is the most common age-dependent neurodegenerative disease, is characterized by the presence of amyloid deposition, neurofibrillary tangles, progressive loss of synapses, and severe cognitive dysfunction (Braak and Braak, 1997; Corriveau et al., 2017). Excessive accumulation of β-amyloid peptides (Aβ) is a widely recognized early event that leads to the development of AD pathologies, including impairments in synaptic functions at various sites (Malenka and Malinow, 2011; Selkoe and Hardy, 2016; Yan et al., 2016). Generation of Aβ requires β-secretase, also called β-site amyloid precursor protein (APP)–cleaving enzyme 1 (BACE1), which cleaves APP to release a soluble N-terminal fragment and a membrane-anchored C-terminal fragment (Hussain et al., 1999; Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999; Lin et al., 2000). Further cleavage of the C-terminal fragment by γ-secretase excises Aβ (Sisodia and St George-Hyslop, 2002; De Strooper et al., 2012). Genetic mutations such as the K670M671 to N670L671 mutation (Mullan et al., 1992) or the A673 to T673 mutation (Jonsson et al., 2012) can either increase or decrease Aβ generation, resulting in early-onset AD or protection against developing AD. Mice completely deficient in BACE1 show nearly abolished Aβ production (Cai et al., 2001; Luo et al., 2001; Roberds et al., 2001), further confirming that BACE1 is an important target for AD treatment.
However, the use of BACE1 inhibition is not without concerns. Mice with BACE1 ablation exhibit abnormal astrogenesis, reduced neurogenesis, hyperactivities, impaired axonal growth and pathfinding, hypomyelination, altered long-term potentiation (LTP), and long-term depression, as well as defects in muscle spindles (see reviews in Vassar et al., 2014; Yan and Vassar, 2014; Barão et al., 2016; Hu et al., 2016). These phenotypes appear to be related to the abolished cleavage of BACE1 cellular substrates such as neuregulin-1 (Nrg1), Jagged 1 (Jag1), close homologue of L1, seizure protein 6, and voltage-gated sodium channel protein β subunits.
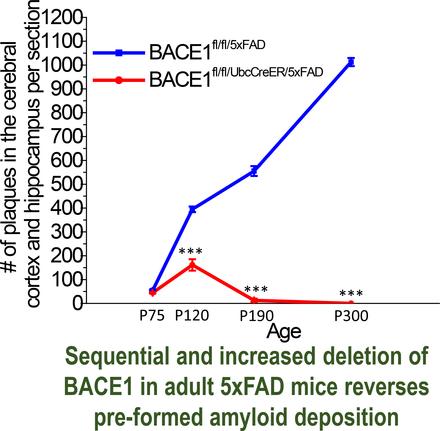
To better understand how BACE1 inhibition in adults will benefit AD patients, we generated homozygous BACE1 flox (fl) mice in which the BACE1 gene can be temporally and tissue-specifically ablated by inducible Cre/lox technology. We bred BACE1 conditional KO mice (BACE1fl/fl mice) with ubiquitin-CreER mice, which express Cre-ER driven by the ubiquitin C promoter in almost all tissues after treatment with tamoxifen (Ruzankina et al., 2007). We found significantly reduced BACE1 expression in adult BACE1fl/fl/UbcCreER mice even before tamoxifen treatment, and ∼50% deletion of BACE1 occurred after postnatal day 60 (P60). Hence, the 5xFAD mouse model was chosen for this study because of the development of amyloid plaques after P60 in this model (Oakley et al., 2006). Strikingly, deletion of BACE1 in adult 5xFAD mice showed a remarkable reversal of amyloid deposition. To our knowledge, this is the first evidence that amyloid plaques can be completely reversed by gradual deletion of BACE1 beginning in early developmental stages. More importantly, the reversal of amyloid deposition in this AD mouse model significantly reduced neuronal loss, and cognitive functions were improved. Hence, this knowledge provides a strong foundation for the concept that BACE1 inhibitors should be administered to humans as early as possible to prevent or reverse amyloid deposition. However, we also demonstrate that caution is warranted, as BACE1 itself is required for optimal cognitive functions….