The restoration of dentine lost in deep caries lesions in teeth is a routine and common treatment that involves the use of inorganic cements based on calcium or silicon-based mineral aggregates. Such cements remain in the tooth and fail to degrade and thus normal mineral volume is never completely restored. Here we describe a novel, biological approach to dentine restoration that stimulates the natural formation of reparative dentine via the mobilisation of resident stem cells in the tooth pulp. Biodegradable, clinically-approved collagen sponges are used to deliver low doses of small molecule glycogen synthase kinase (GSK-3) antagonists that promote the natural processes of reparative dentine formation to completely restore dentine. Since the carrier sponge is degraded over time, dentine replaces the degraded sponge leading to a complete, effective natural repair. This simple, rapid natural tooth repair process could thus potentially provide a new approach to clinical tooth restoration.
Introduction
Dentine is a vital tooth mineral that is produced by highly specialised mesenchymal cells called odontoblasts. When tooth mineral is compromised either following trauma or infection (caries), the inner cellular soft pulp tissue can become exposed to the external environment and become infected. Clinical repair of tooth damage currently involves the use of mineral aggregates that are used to fill the space in dentine created following removal of decay or trauma1,2,3,4,5. When the soft inner pulp tissue is exposed, a natural repair process is activated that involves the mobilisation of resident mesenchymal stem cells to differentiate into new odontoblast-like cells that secrete a form of tertiary (reparative) dentine6,7,8,9. The reparative dentine produced forms a thin band of dentine (dentine bridge) that serves to protect the pulp from infection by sealing the tooth pulp from the external environment. Unfortunately, natural reparative dentine formation is insufficient to effectively repair large lesions, such as those involving the loss of dentine after caries removal and hence artificial mineral aggregates are used to fill the tooth and replace the lost dentine.
The activation of Wnt/β-cat signalling is an immediate early response to tissue damage and appears to be essential for stimulating the cellular-based repair in all tissues10,11,12,13. Axin 2 is a negative regulator and also a downstream target of this signaling pathway. A key cytoplasmic component of Wnt/β-cat signal transduction is the enzyme, glycogen synthase kinase 3 (GSK-3) that in the absence of Wnt ligand/receptor binding, phosphorylates β-catenin and Axin leading to ubiquitination and degradation. In the presence of Wnt ligands, GSK-3 activity is inhibited allowing β-catenin to enter the nucleus where it interacts with Lef/Tcf transcription factors to regulate expression of target genes, that include Axin214. Having first confirmed that Axin 2 expression and hence Wnt/β-cat signaling is upregulated following tooth damage we reasoned that addition of Wnt signaling agonists may provide an effective way to stimulate reparative dentine formation and thus restore lost dentine following caries removal with naturally-generated new dentine (Fig. S1). Numerous small molecule inhibitors of glycogen synthase kinase 3 (GSK3) have been developed and shown to efficiently upregulate Wnt activity in different experimental contexts and in one case, that of Tideglusib (NP-12, NP03112), are in clinical trials for the treatment of neurological disorders such as Alzheimers disease15,16,17,18,19,20,21. We tested the ability of three small molecule GSK3 inhibitors, BIO (2′Z,3′E)-6-Bromoindirubin-3′-oxime), CHIR99021(6-[[2-[[4-(2,4-Dichlorophenyl)-5-(5-methyl-1H-imidazol-2-yl)-2 pyrimidinyl]amino]ethyl]amino]-3-pyridinecarbonitrile) and Tideglusib (4-Benzyl-2-(naphthalen-1-yl)-[1,2,4]thiadiazolidine-3,5-dione) to stimulate tertiary dentine following experimentally induced pulp exposure22,23,24. As a delivery vehicle we used a commercially-available, clinically-approved collagen sponge, Kolspon.
Results
Effective concentrations and cytotoxicity testing
17IA4 mouse dental pulp cells were incubated with a range of concentrations of the three inhibitors and cytotoxicity analysed with the MTT assay after 24 h in culture (Fig. 1A–C)25,26. The highest concentration of inhibitor that was not cytotoxic was used in separate assays with the same cells and levels of Axin2 measured by qPCR in the first 24 h of culture. Increased Axin2 expression was observed after 30 mins and this reached a maximum after 1 hr (Fig. 1D). BIO induction of Axin2 expression was four fold greater than both CHIR99021 and Tideglusib, each of which showed similar levels of induction.
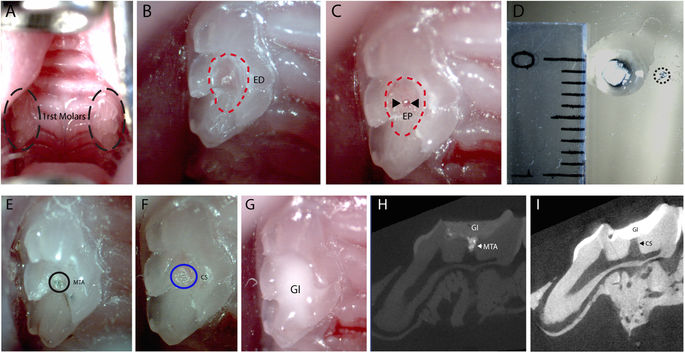
To test the induction of Axin2 in vivo, experimental tooth damage was created, by drilling and making 0.13 mm holes in mouse maxillary first molars to expose the pulp (Fig. 2). Pieces of Kolspon were cut to size and soaked in solutions of the three inhibitors before being physically placed into the holes, in contact with the pulp. A glass ionomer cement was used to cover the sponge and protect the tooth (Fig. 2G). Treated teeth were removed after 24 h along with controls consisting of untreated teeth, MTA only and collagen sponge with no inhibitor. Pulp cells were extracted and tested for expression of Axin2 by qPCR (Fig. 1E). Expression of Axin2 was found to be 3 fold higher in inhibitor treated pulp cells when compared to controls (Fig. 1E). Significantly MTA showed no effect on Axin2 expression over controls suggesting current protocols do not lead to enhanced activation of Wnt signalling. After 5 days post treatment, Axin 2 expression levels were the same in with MTA and agonist treatments but these results were compounded by the fact that newly forming odotonblast-like cells express high levels of Axin 2 (Figs S1–2).
Reparative dentine formation
Having established that the experimental model of tooth damage and pulp exposure provided a way of delivering small molecules that were able to affect pulp cell gene expression in a reproducible way, we used this method to examine the effect on the formation of reparative dentine. Maxillary molars were drilled and sponges inserted and left for 4–6 weeks before removal. Micro-computed tomographic (μCT) scanning was used to visualise and quantify mineral deposition at the drill site. Analysis at both 4 and 6 weeks revealed increased mineralisation with all three agonists when compared to controls with no obvious increases between 4 to 6 weeks (Fig. 3K,L). These increases were statistically significant for BIO, CHIR and Tideglusib both at 4 and 6 weeks. Overall the mineralisation with the inhibitors was on average 2 times greater than in the sponge alone control and 1.7 times greater than with MTA treatment. Following decalcification, sections were made through each molar and stained to reveal new dentine formation. The sections confirmed the μCT data showing that when teeth were treated with GSK-3 inhibitors, more reparative dentine was formed at the injury site than with collagen sponge or MTA (Fig. 4). Moreover, the new dentine formed with the new conditions presented as dense dentine localised centrally to the injury site, revealing no remaining collagen sponge where the dentine was formed. Interestingly, by 6 weeks of treatment, the reparative dentine secreted when teeth were treated with BIO, CHIR, and Tideglusib filled the whole injury site from occlusal to pulp chamber roof (Fig. 4H–J). Most importantly, dental pulp remained vital compared to controls consisting of exposed pulp with no capping, glass ionomer only showed no evidence of reparative dentine formation and severely hypoplastic pulp (Figs 4H–J; S3).
Discussion
Modern dental practice for carious lesions aims to remove decay and restore tooth structure by using mineral aggregate filling materials. Preservation of undamaged dentine forms an integral part of this practice since maintenance of as much of the natural mineral as possible is deemed important for tooth vitality. Mineral aggregates such as MTA and Biodentine are reported to aid the formation of tertiary dentine, although the deposition of this dentine is not at the sites of damage but rather internal in the pulp space27,28,29. In addition the non-biodegradeable nature of these materials means that the full mineral volume is never restored. If a simple method can be developed that acts to enhance the natural processes of dentine restoration by stimulating tertiary dentine formation, then large injuries that would certainly lead the dental pulp to undergo necrosis could be repaired by enabling reparative dentine to be formed at the site of damage. The activation of Wnt/βCat signalling as a universal immediate-early response to tissue damage provides a potential route for enhancing natural repair by overstimulating this pathway30. Wnt/βcatenin signalling has thus emerged as a major target in tissue regeneration and repair and this pathway activity can be stimulated in a number of different ways. We chose small molecule agonists as a simple, cost-effective method that is supported by substantial existing experimental data and clinical use. In our damage model system we did not observe any effects of MTA on the enhancement of Wnt signalling activity and although it may be acting via other pathways it seems likely that any positive action on mineralisation is as result of providing mineral ions. We developed a method that uses an already clinically-approved biomaterial (collagen sponge – Kolspon) as a delivery vehicle for small molecule GSK-3 inhibitors that act as Wnt agonists. Both BIO and CHIR99021 have been extensively used experimentally to elevate Wnt activity while Tideglusib is in clinical trials for systemic use in the treatment of neurological disorders include Alzheimers disease15,16,17,18,19,20,21. Since upregulated Wnt activity in response to damage is an immediate early response we aimed to achieve rapid release of small molecule agonists and reasoned a sponge was the most effective way of ensuring this. All three agonists showed significantly increased mineralisation at the site of damage compared to the use of the sponge alone or MTA treatment. More significantly the localization of the reparative dentine formed indicated that with the treatments, the mineral replaced the biodegradable sponge and restored the cavity in the dentine made by the burr. With MTA the cavity remains permanently filled with mineral aggregate and this non-degradable material can only affect reparative dentine formation on the pulp chamber aspect.
An important consideration is the effect Wnt agonists may have following their release into the circulation. The small localised doses of these agonists used were effective at increasing the formation of reparative dentine to the extent that almost complete repair of the lesion was observed after 6 weeks. These doses are substantially lower that those used in clinical trials of Tideglusib where 500–1000 mg were delivered systemically daily for 26 weeks20. We used a maximum of 21 pg of Tideglusib on the sponges and thus even if 100% of the drug on the sponge is released within a few hours, the maximum systemic concentration, assuming all the drug enters the circulation, would be no more than 21 pg in 1.5 ml. Mouse blood volume is approximately 3000 times smaller than that of a human and thus the mouse dosage in the circulation is equivalent to 63 ng in the human circulation, or 1000 times lower than used in clinical trials. Extrapolating the size of a mouse first molar to that of a human suggests that an equivalent lesion would require around 10 times more reparative dentine formation and thus the anticipated concentrations of Tideglusib required for human tooth repair would be well below that already tested in clinical trials.
Small molecule Wnt signalling agonists delivered via a biodegradable collagen sponge provide an effective repair of experimentally-induced deep dental lesions by promotion of reparative dentine formation. The simplicity of this approach makes it ideally translatable into a clinical dental product for treatments requiring dentine restoration and pulp protection that are currently treated with non-organic cements…….