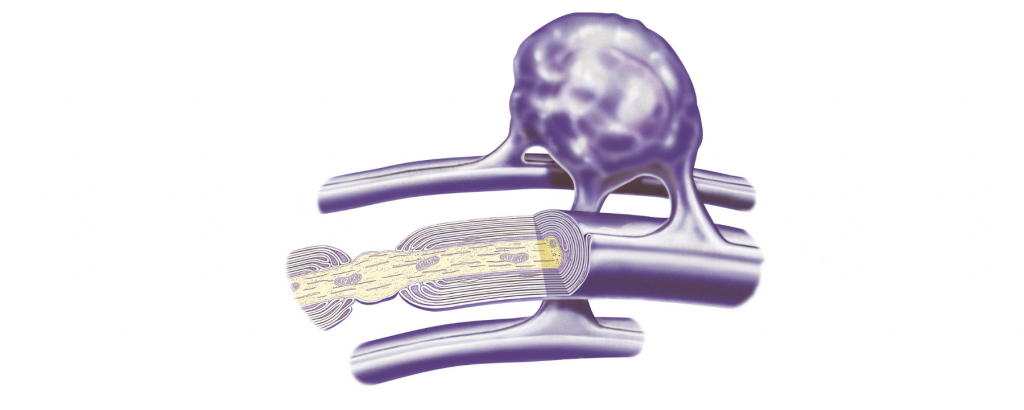
A mesh-like network of cells within mice’s skin plays a previously unknown role in pain perception, researchers reported today (August 16) in Science. The scientists have called the intricate structure a “new sensory organ,” responsible for reacting to mechanical trauma such as being poked with a needle or smacked with a bat.
“Our study shows that sensitivity to pain does not occur only in the skin’s nerve fibres, but also in this recently-discovered pain-sensitive organ,” says coauthor Patrik Ernfors, a molecular neurobiologist at the Karolinska Institute, in an announcement. “The major question for us now is whether these cells are actually the cause for certain kinds of chronic pain disorders,” he adds in an interview with The Guardian.
It’s been established that humans primarily sense pain through free nerve endings of specific sensory cells. Unlike other nerve cells, these aren’t insulated in a protective coating of myelin, but instead are enveloped by glia known as Schwann cells that connect them to nearby cells, according to Gizmodo. The new study reveals that certain Schwann cells, which lie beneath the outermost layer of skin, reach out spindly extensions that wrap around nerve endings in the epidermis, form a web-like network, and contribute to pain signaling.
“In the pain field, we talk about free nerve endings that are responsible for pain sensation. But actually they are not free,” Ernfors tells The Guardian. “It is a two-cell receptor organ: the nerve and Schwann cell together.”
The researchers manipulated the function of these nociceptive Schwann cells in mice using optogenetics. When the cells were activated with light stimulation, the mice licked and guarded their paws as if experiencing pain. The scientists also tested what happened when the mice experienced a pinprick, heat, or cold after the network was activated or deactivated. Following light stimulation, the animals reacted more strongly to all three painful stimuli than before, but when the network was disabled, their ability to sense mechanical pain diminished. The results suggest.
Upon activation, the network directs electrical impulses through the nervous system that give rise to feelings of pain, according to the institution’s announcement. The longer the cells remain active, the more surrounding neurons begin to fire, according to The Guardian.
“We have not studied humans yet. However, considering that all previously known sensory organs found in mouse also exist in humans, it is possible if not likely that it does exist also in the human skin,” Ernfors tells Gizmodo in an email. He adds that, because the newly discovered cells sense mechanical pressure, they could play a role in mechanical allodynia, a condition where normally non-painful stimuli such as the brush of fabric or a soft touch hurt terribly.
The researchers plan to study nociceptive Schwann cells further to determine how they respond to pain, mechanistically, and how they contribute to animal models of chronic pain.
“If borne out by subsequent studies, this paper will be a paradigm shift showing that pain-sensitive nerve cell terminals are not in fact always directly driven by a painful stimulus but instead can be driven by associated [Schwann] cells,” says Peter McNaughton, an expert in sensory physiology and pharmacology at King’s College London, to The Guardian.