Highlights
•Somatic cell nuclear transfer (SCNT) using fetal fibroblasts yielded two live monkeys
•Epigenetic modulators promoted development and pregnancy rate of SCNT embryos
•SCNT using adult cumulus cells yielded live births of monkeys that were short-lived
•Genetic analysis confirmed the clonal origin of the SCNT monkey offspring
Summary
Generation of genetically uniform non-human primates may help to establish animal models for primate biology and biomedical research. In this study, we have successfully cloned cynomolgus monkeys (Macaca fascicularis) by somatic cell nuclear transfer (SCNT). We found that injection of H3K9me3 demethylase Kdm4d mRNA and treatment with histone deacetylase inhibitor trichostatin A at one-cell stage following SCNT greatly improved blastocyst development and pregnancy rate of transplanted SCNT embryos in surrogate monkeys. For SCNT using fetal monkey fibroblasts, 6 pregnancies were confirmed in 21 surrogates and yielded 2 healthy babies. For SCNT using adult monkey cumulus cells, 22 pregnancies were confirmed in 42 surrogates and yielded 2 babies that were short-lived. In both cases, genetic analyses confirmed that the nuclear DNA and mitochondria DNA of the monkey offspring originated from the nucleus donor cell and the oocyte donor monkey, respectively. Thus, cloning macaque monkeys by SCNT is feasible using fetal fibroblasts.
Introduction
Cloning of animals by somatic cell nuclear transfer (SCNT) has been achieved in 23 mammalian species (see review Rodriguez-Osorio et al., 2012), including sheep (Wilmut et al., 2007), mouse (Wakayama et al., 1998), cattle (Kato et al., 1998), pig (Polejaeva et al., 2000), cat (Shin et al., 2002), rat (Zhou et al., 2003), and dog (Lee et al., 2005). As species closer to humans, non-human primates are ideal animal models for studying physiological functions unique to primates and for developing therapeutic treatments of human diseases (Izpisua Belmonte et al., 2015, Jennings et al., 2016). Animal models with genetic uniformity are often desirable (Schramm and Paprocki, 2004), but an inbreeding approach as used in generating rodent models is not practical for non-human primates because of their long generation time (Smedley et al., 2002), although the latter could be shortened by speeding sperm maturation with testicular xenografting (Liu et al., 2016b). Cloning of non-human primates by SCNT has failed to generate live offspring so far (Mitalipov et al., 2002, Mitalipov et al., 2007, Ng et al., 2004, Simerly et al., 2003, Sparman et al., 2010). In view of the fact that live monkeys could be generated using nuclear transfer of early blastomeres (Meng et al., 1997), previous failure of SCNT appears to be caused by inappropriate reprogramming of the somatic nucleus for supporting the development of transplanted embryos.
Histone deacetylase inhibitors such as trichostatin A and scriptaid have been used to improve the efficiency of mammalian SCNT in several species, including mouse, bovine, pig, monkey, and human (Akagi et al., 2011, Kishigami et al., 2006, Sparman et al., 2010, Tachibana et al., 2013, Zhao et al., 2009). Furthermore, reprogramming-resistant regions (RRRs) that are enriched for histone 3 lysine 9 trimethylation (H3K9me3) modification were identified in SCNT embryos. Expression of human H3K9me3 demethylase Kdm4d/4a could reduce the H3K9me3 level and significantly improve the efficiency of both mouse and human SCNT (Antony et al., 2013, Chung et al., 2015, Liu et al., 2016a, Matoba et al., 2014), suggesting that RRRs are conserved among mammalian species. In this study, we applied both histone demethylase Kdm4d mRNA and histone deacetylase inhibitor trichostatin A (TSA) to the cloning of macaque monkeys. We performed SCNT using both fetal monkey fibroblasts and adult monkey cumulus cells and successfully produced live birth of monkey offspring carrying nuclear DNA of the donor cell and mitochondria DNA of the oocyte donor monkey. Monkey neonates generated using fetal fibroblasts were healthy, whereas those generated using adult cumulus cells survived only briefly after birth. Since fetal fibroblasts could be genetically modified efficiently in vitro and properly screened for precise gene editing (Gao et al., 2017, Lai et al., 2016, Rogers, 2016) prior to SCNT, our results pave the way for the generation of genetically uniform monkey models for basic research and biomedical applications.
Results
Optimization of SCNT Methods
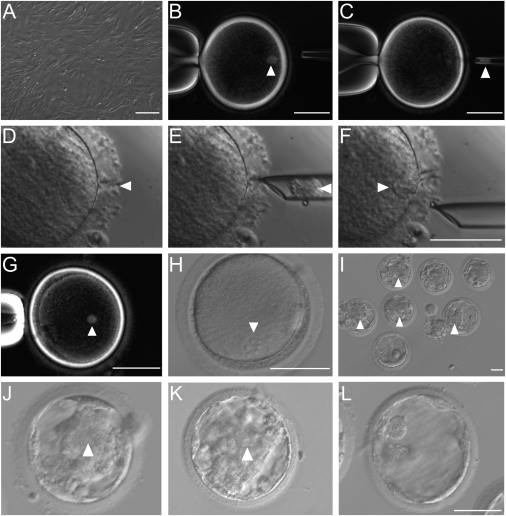
We first optimized the SCNT protocol, including polarized-light imaging for the removal of spindle-chromosome complex from the oocyte, incubation of the nuclear donor cell with viral envelope isolated from the Hemagglutinin Virus of Japan (HVJ-E, Tachibana et al., 2010), and facilitation of donor cell insertion into the perivitelline space with laser lesion of zona pellucida (Figure 1A–1F ). Clear spindle-like structure was detected after the fusion of the fetal monkey fibroblast with the enucleated oocyte (Figures 1G and S1A). About 1–2 hr after the fusion, “reconstructed” oocytes were activated with ionomycin and 6-dimethylaminopurine (I/D). All activated embryos showed a single pronucleus (Figures 1H and S1B) and were further cultured for in vitro development. A small percentage (4/30, or 13.8%) of I/D-activated SCNT embryos developed to the blastocyst stage. To improve the developmental potential of SCNT embryos, we treated another group of SCNT embryos (I/D/T) with 10 nM of the histone deacetylase inhibitor trichostatin A (TSA) (Kishigami et al., 2006) for 10 hr during and after I/D activation. We found a similarly low blastocyst rate (5/31, or 16.7%), but the quality of blastocysts was improved. For the I/D-activated group, we found no or little inner cell mass (ICM), but prominent ICM was observed in 2/5 of the blastocysts in the I/D/T group (Figures 2A, 2B , S1C, and S1D).
Based on the recent progress in studying epigenetic modification of transferred nuclear DNA in mammalian cloning by SCNT (Matoba et al., 2014), we injected exogenous human Kdm4d mRNA to the SCNT embryos after the activation under I/D/T condition (I/D/T/K). We found that a much higher percentage (17/38, or 44.7%) of Kdm4d mRNA-injected SCNT embryos derived from fetal monkey fibroblasts were able to develop into blastocysts, among which a large fraction (11/17, or 64.7%) showed prominent ICM similar to the ICM in embryos obtained by intracytoplasmic sperm injection (Figures 1I–1L, 2A, and 2B). We also tested the effect of Kdm4d mRNA injection on SCNT embryos derived from cumulus cells of adult female monkeys (from which the oocytes were obtained) and found that all SCNT embryos showed a single pronucleus after activation under I/D/T condition (Figures S2A–S2D). The majority of them (24/33, or 72.7%) developed into blastocysts, most of which (15/24, or 62.5%) showed prominent ICM. By contrast, in the absence of Kdm4d mRNA injection, only 5% (1/20) of SCNT embryos derived from cumulus cells developed into blastocysts, none of which showed prominent ICM (Figures 2C, 2D, S2E, and S2F).
To further understand the mechanism by which Kdm4d mRNA improved the development of monkey SCNT embryos, RNA sequencing (RNA-seq) was performed on cumulus cells, 4- and 8-cell embryos obtained by ICSI, and 8-cell SCNT embryos (derived from cumulus cells) with and without Kdm4d mRNA injection. Analysis of RNA-seq datasets of monkey ICSI embryos at 4- and 8-cell stage (n = 4 embryos each) led to identification of 3,997 regions (20–160 kb) that were expressed at least 5-fold higher (FC > 5) in 8-cell embryos as compared to 4-cell embryos (Data S1), indicating massive upregulation of genes during early embryonic development. Comparison of these regions between 8-cell ICSI embryos and 8-cell SCNT embryos without Kdm4d mRNA injection showed 2,465 RRRs (FC > 5) in SCNT embryos (Data S1). By contrast, 2,178/2,465 RRRs were markedly upregulated (FC > 2) in Kdm4d mRNA-injected SCNT embryos (Figure 2E, Data S1). The effect of Kdm4d mRNA expression in removing the histone methylation site H3K9me3 of SCNT embryos was further confirmed by immunostaining of H3K9me3 (Figure 2F), showing that the level of H3K9me3 was high in control one-cell SCNT embryos but greatly reduced in those injected with Kdm4d mRNA.
To identify candidate genes that were repressed by H3K9me3 and may be responsible for the poor development of monkey SCNT embryos, we further examined the RNA-seq data and found that some developmental pluripotency-associated genes such as Dppa2, Dppa4, and Myc were repressed by H3K9me3 in monkey SCNT embryos. Other repressed genes found in mouse SCNT embryos, such as Zscan4 and Polr3h, were also identified in monkey SCNT embryos. A more complete list of genes identified is shown in Data S1. Further studies on directly manipulating these genes in SCNT embryos are needed to confirm their roles in facilitating embryonic development (Matoba et al., 2014). Taken together, the expression of H3K9me3 demethylase Kdm4d significantly improved epigenetic reprogramming in monkey SCNT embryos similar to that found in other species (Chung et al., 2015, Matoba et al., 2014). Thus, Kdm4d mRNA injection was used in all subsequent experiments in the present study.
SCNT Using Fetal Monkey Fibroblasts
Fetal fibroblasts in primary culture derived from an aborted female cynomolgus monkey fetus were prepared by standard methods (see STAR Methods) and used for SCNT. These cells were chosen for their potential in obtaining a large number of nuclei with uniform genetic background. Using the SCNT protocol described above, a total of 109 Kdm4d mRNA-injected SCNT embryos under I/D/T condition were obtained using 127 MII-stage oocytes, and 79 of them (between 2-cell to blastocyst stage) were transferred to 21 cynomolgus female surrogates (Sun et al., 2008) (Table 1 and Data S2). The choice of the embryo transfer time was based on the number of SCNT embryos prepared and that of surrogates available at the time. Pregnancy was confirmed in 6 surrogates by ultrasound examination one month later, 4 of them carried 5 fetuses (one twin), and the other 2 carried only gestational sacs (GS, Figures 3C and 3D ). Among the 4 pregnancies, 2 aborted at the early gestation stage (within two months), and 2 developed beyond 140 days (Figure 3E and Data S2). Two live babies were obtained at full term (155 and 141 days) by caesarean section. The newborn baby monkeys, named Zhong Zhong (ZZ) and Hua Hua (HH) (Figures 4A and 4B ), survived in good conditions under human feeding and care for 50 and 40 days, respectively, at the time of manuscript resubmission. In comparison with newborn monkeys obtained by natural fertilization, they showed no hypertrophy of umbilical cord at birth, normal postnatal temperature regulation, and exhibited sucking reflex during feeding, as well as normal growth rate in body weight and head circumference (Movie S1).
SCNT Using Adult Monkey Cumulus Cells
We also used adult cumulus cells of female monkeys (from which the oocytes were obtained) as donor cells for SCNT. A total of 192 SCNT embryos obtained from 290 MII stage oocytes under I/D/T activation condition, were injected with Kdm4d mRNA at the pronuclear stage, and 181 of them (at 2-cell to blastocyst stage) were transferred to 42 monkey female surrogates (Table 1 and Data S2). Pregnancy was confirmed in 22 surrogates, 12 of them carried 17 live fetuses, and the rest yielded only GS (Figures 3A–3D). Among the 12 pregnant surrogates with fetuses, 8 aborted at early gestation stage (within 2 months) and 2 aborted on day 84 and 94. The last two pregnancies developed beyond 130 days and yielded two live births (infants A and B) via caesarean section on day 135 and 137, respectively (Figure 3E, Data S2). Infant A showed normal head circumference but impaired body development at birth and died 3 hr later due to apparent respiratory failure. Infant B had apparent normal head and body development and showed normal breathing and food and water intake but died 30 hr later with respiratory failure (see Data S4).
Genetic Analyses of Monkeys Generated by SCNT
Single-nucleotide polymorphism (SNP) and short-tandem repeat (STR) analyses (Penedo et al., 2005, Tachibana et al., 2009) were performed to examine the mitochondria and nuclear DNAs of monkey babies obtained by SCNT. The STR analysis of 27 loci demonstrated that the nuclear DNAs of ZZ and HH ear tissues completely matched that of the fetal fibroblast donor, and three example loci are shown in Figure 4C (complete information on 27 loci are shown in Data S3). The SNPs of the ND3 gene of mitochondria DNA for the ear tissue obtained from ZZ and HH were identical to that of their respective oocyte donor monkeys (examples shown in Figures 4D and 4E; more examples are shown in Figure S3) due to the overwhelming abundance of oocyte mitochondria in comparison to that of somatic cell used for SCNT. Similar SNP and STR analyses performed on the ear tissues of deceased infants A and B obtained by SCNT using monkey cumulus cells also confirmed the origin of the mitochondria and nuclear DNAs from their respective oocyte donor monkey and cumulus cells obtained from the latter (Figure S4 and Data S3). These genetic analyses fully confirmed the clonal origin of the monkeys generated by our SCNT method.
Discussion
In this study, we have generated two healthy cynomolgus monkeys by the SCNT method using fetal monkey fibroblasts. We attributed the success of cloning of a non-human primate by SCNT to the optimization of the nuclear transfer protocol, the use of fetal cell nuclei, and epigenetic modifications by Kdm4d and TSA, and all of them together greatly improved the quality of blastocyst development and pregnancy rate. Genetic analysis fully confirmed the clonal origin of the monkeys generated by SCNT. This study demonstrated that cloning of non-human primates is feasible by SCNT using fetal somatic cells, which could be efficiently modified by genetic editing and screening in vitro (Gao et al., 2017, Lai et al., 2016, Rogers, 2016). Such cloning allows the production of genetically uniform monkeys as animal models for basic research in primate biology and for studying human disease mechanisms and therapeutic treatments.
Previous studies on monkey SCNT using adult fibroblasts (Sparman et al., 2010) and cumulus cells (Ng et al., 2004) have yielded pregnancies up to 80 days. The main obstacle to the live birth appeared to be poor reprogramming of transferred nuclei for supporting embryonic development. In this study, we demonstrated the effectiveness of H3K9me3 demethylase Kdm4d and histone deacetylase inhibitor TSA in promoting normal blastocyst formation, pregnancy rate, and fetal development. Immunostaining of Kdm4d mRNA-injected one-cell embryos showed that H3K9me3 sites were greatly reduced (Figure 2F), and transcriptome analysis further confirmed that Kdm4d treatment indeed promoted the upregulation of RRRs of the embryo’s genome at the early blastomere stage (Figure 2E). In addition to the use of H3K9me3 demethylase and histone deacetylase inhibitor, several other approaches such as impeding Xist gene expression of the active X chromosome (Inoue et al., 2010), embryo aggregation (Boiani et al., 2003), trophectoderm replacement (Lin et al., 2011), and H3K4me3 demethylase injection (Liu et al., 2016a) were also found to improve the development of mouse SCNT embryos. Previous studies showed that caffeine, electric stimulation (Tachibana et al., 2013), and translation inhibitor puromycin (Yamada et al., 2014) could facilitate the production of human embryonic stem cells via SCNT methods. All these additional approaches could be used for further improving the efficiency of monkey SCNT. Finally, we noted that the lower efficiency in SCNT using adult cumulus cells as compared to fetal fibroblasts could be attributed to the fact that reprogramming of adult nuclei is less efficient than fetal nuclei or, alternatively, to the difference in the somatic cell type. More extensive studies of reprogramming adult somatic nuclei are required for the future use of SCNT on adult somatic cells.
Our choice of using fetal fibroblasts as donor cells for SCNT is based on the consideration of not only reprogramming potential of fetal cells, but also their potential application for genetic editing. Although recent advances in gene editing methods, e.g., CRISPR/Cas9 technology, could achieve efficient gene knockout or knockin monkey embryos (Niu et al., 2014, Cui et al., 2018, Yao et al., 2018), mosaicism in gene editing and off-target effects remain problematic for direct gene editing of monkey embryos. In vitro screening of gene-edited fibroblasts prior to SCNT could circumvent some of these problems and allow the generation of a large number of cloned monkeys with uniform genetic background. This is particularly relevant for developing monkey models for human diseases with defined genetic defects that could be used for studying disease mechanisms and testing potential therapeutic treatments. Basic studies on the genetic basis of primate-specific traits will find genetically uniform clones of non-human primates a useful laboratory animal model.