Highlights
- •Crystal structure of the human 5-HT2B receptor bound to LSD is determined
- •LSD shows unexpected binding configuration in the orthosteric site
- •LSD has extremely slow on and off rate at 5-HT2B and 5-HT2A receptors
- •Accelerated LSD kinetics selectively reduce arrestin signaling at 5-HT2B and 5-HT2A
Summary
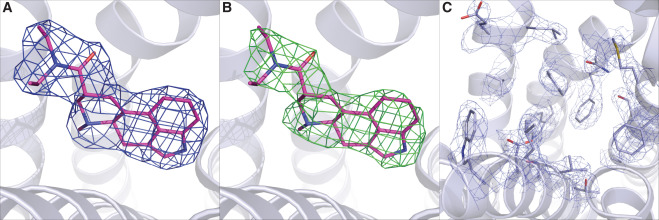
The prototypical hallucinogen LSD acts via serotonin receptors, and here we describe the crystal structure of LSD in complex with the human serotonin receptor 5-HT2B. The complex reveals conformational rearrangements to accommodate LSD, providing a structural explanation for the conformational selectivity of LSD’s key diethylamide moiety. LSD dissociates exceptionally slow from both 5-HT2BR and 5-HT2AR—a major target for its psychoactivity. Molecular dynamics (MD) simulations suggest that LSD’s slow binding kinetics may be due to a “lid” formed by extracellular loop 2 (EL2) at the entrance to the binding pocket. A mutation predicted to increase the mobility of this lid greatly accelerates LSD’s binding kinetics and selectively dampens LSD-mediated β-arrestin2 recruitment. This study thus reveals an unexpected binding mode of LSD; illuminates key features of its kinetics, stereochemistry, and signaling; and provides a molecular explanation for LSD’s actions at human serotonin receptors.