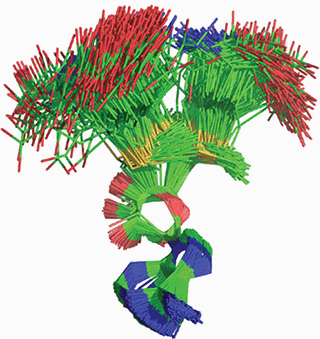
A small fraction of proteins have an unusual conformation in which the backbone forms a knot. An example is a bacterial enzyme, TrmD, that transfers a methyl group from S-adenosyl methionine (AdoMet) to a guanine nucleotide that is conserved in many transfer RNAs (tRNAs). This methyl transfer ensures accurate protein synthesis. Christian et al. combine structural, mutagenesis, and computational studies to examine the role of the protein knot in catalysis. They show that the knot binds the AdoMet in a bent conformation oriented for methyl transfer. Despite its constrained topology, the knot undergoes complex dynamics that couple AdoMet binding to tRNA binding and facilitate catalysis.