Abstract
Tissue injury to skin diminishes miR-200b in dermal fibroblasts. Fibroblasts are widely reported to directly reprogram into endothelial-like cells and we hypothesized that miR-200b inhibition may cause such changes. We transfected human dermal fibroblasts with anti-miR-200b oligonucleotide, then using single cell RNA sequencing, identified emergence of a vasculogenic subset with a distinct fibroblast transcriptome and demonstrated blood vessel forming function in vivo. Anti-miR-200b delivery to murine injury sites likewise enhanced tissue perfusion, wound closure, and vasculogenic fibroblast contribution to perfused vessels in a FLI1 dependent manner. Vasculogenic fibroblast subset emergence was blunted in delayed healing wounds of diabetic animals but, topical tissue nanotransfection of a single anti-miR-200b oligonucleotide was sufficient to restore FLI1 expression, vasculogenic fibroblast emergence, tissue perfusion, and wound healing. Augmenting a physiologic tissue injury adaptive response mechanism that produces a vasculogenic fibroblast state change opens new avenues for therapeutic tissue vascularization of ischemic wounds.
Introduction
Single cell RNA sequencing has propelled advances in understanding basal and activated fibroblast subset heterogeneity that varies with age1, disease2,3, injury4, and organs5,6. Certain myocardial and skeletal muscle fibroblast subsets become activated following ischemic injury to serve as pro-angiogenic fibroblasts that enhance tissue perfusion and reparative processes3,7. However, it is unclear if pro-angiogenic fibroblast subsets contribute to new blood vessel formation. Here, we report on the maiden identification of a physiological and inducible vasculogenic fibroblast which serves a critical role in the initiation of functional tissue vascularization.
Results
Inhibition of miR-200b in cultured dermal fibroblasts
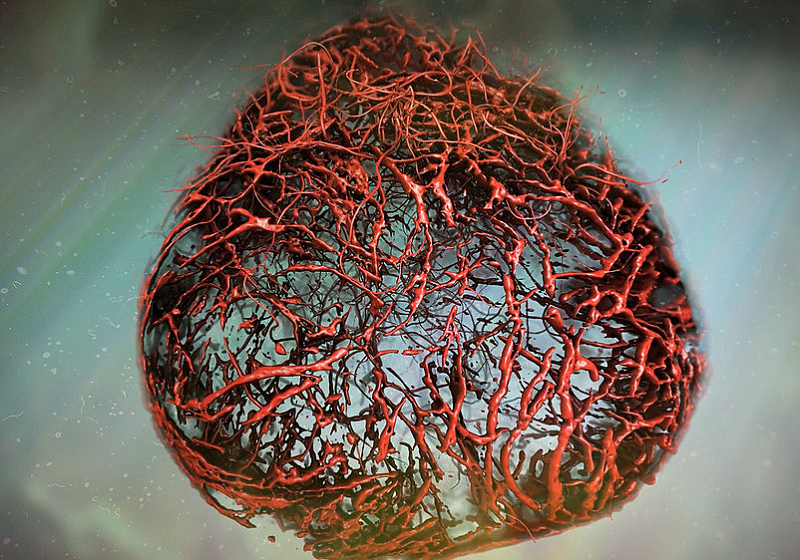
MiR-200b abundance is sharply lowered at the wound-edge of patient skin8 (Supplementary Fig. 1a). Murine skin wounding induced a transient 7-day deficit of miR-200b in dermal fibroblast rich tissue (Supplementary Fig. 1b) but, not in K14 + epithelial or F4/80+ macrophage elements (Supplementary Fig. 1c). To understand the significance of lowered miR-200b abundance in fibroblasts, anti-miR-200b oligonucleotide was delivered in vitro to human adult dermal fibroblasts (HADF) (Fig. 1a) via in vitro TNT9 with high efficiency (86.21 ± 4.05%; n = 5). To interrogate single cell HADF transcriptome complexity, all cells from anti-miR-200b treated samples (days 1, 3, 5, and 7) were compared to pre-treated reference control HADF cells (day 0) (Fig. 1b). The initial dataset contained 40,212 cells and the 36,308 cells that met quality control parameters underwent downstream analysis. Unsupervised clustering identified 4 clusters (Fig. 1b). Compositional analysis revealed cluster 1 cells increased > 10-fold in frequency over 7 days post-transfection but clusters 0, 2, and 3 decreased over time (Fig. 1b). Differentially expressed genes significantly increased over time (Supplementary Fig. 1d) indicative of an activated transcriptionally responsive process10. Reactome pathway enrichment for the differentially expressed genes revealed that collagen formation/degradation pathways were among several pathways significantly downregulated over time (Supplementary Fig. 1e, f). However, all 4 clusters displayed well-known fibroblast marker gene transcripts11,12,13 such as CD9014, FSP1 (S100A4)15, vimentin16, and fibroblast activation protein alpha 1 (FAP)17 (Fig. 1c). Among the 4 clusters, cluster 1 uniquely and temporally lost collagen gene transcripts (Fig. 1d, e), while significantly gaining endothelial gene transcripts (Fig. 1e, Supplementary Fig. 2a, b), including numerous VEGF pathway genes that play important roles in angiogenesis and vasculogenesis14,18,19. Quantitative validation of endothelial gene transcripts in cluster 1, was confirmed without Col1A1 transcript abundance change (Fig. 2a). As an additional control, HADF cells were treated with a control or anti-miR-200b oligonucleotide by TNT. A significant difference in endothelial transcripts at 7 days post-transfection was detected for all clusters combined and for cluster 1, clarifying that the induced gain in endothelial transcripts was not related to transfection of any oligonucleotide into HADF (Supplementary Fig. 2c, d). Analysis of cell surface proteins in clusters 0-4 (Supplementary Fig. 3a) identified cluster 1 as beta 2 microglobulin high (B2Mhi) and integrin alpha 2 low (ITGA2lo) while all remaining clusters were B2Mlo and ITGA2hi. Flow cytometric sorting of these subsets identified the B2MhiITGA2lo cells displaying the lowest miR-200b abundance and highest FLI1 expression among the anti-miR-200b post-transfected day 3 HADF (Supplementary Fig. 3b, c). No significant differences were observed in cell cycle genes when compared to cluster 0 and 1 at 1- and 7-days post-transfection (Supplementary Fig. 3e). Akin to the function of endothelial cells, cluster 1 cells also displayed endothelial nitric oxide synthase (eNOS) expression (Fig. 2b), ingested acetylated-low density lipoprotein (ac-LDL) (Fig. 2c), and formed branching tubular structures when plated on Matrigel (Fig. 2d). These results reflected acquisition of angiogenic properties in treated fibroblasts but did not formally prove development of lumenized vessels de novo from endothelial precursors20. Thus, commercially available HADF expressing green fluorescent protein (GFP) were treated with anti-miR-200b or control oligonucleotides and suspended along with tdTomato+ cord blood endothelial colony forming cells (ECFC) (1:1 ratio with ECFC as positive control) in collagen gels, cultured in vitro for 48 h, and implanted within the subcutaneous space of immunodeficient mice. Four weeks later, confirmation of intravenously delivered lectin perfused chimeric capillary vessels comprised of human vasculogenic fibroblasts (VF) and some tdTomato+ ECFC cells were detected in 6 of 9 test mice (Fig. 2e, f). No HADF-GFP control cells formed vessels (n = 6). Thus, cultured HADF, treated with TNT delivered anti-miR-200b oligonucleotides displayed acquisition of the capacity to form perfused human blood vessels for at least one month in immunodeficient mice. However, these COL1A2 + VEGFR2 + VF continued to remodel and contract 3D collagen gels as effectively as control HADF cells in contrast to equal numbers of HMEC control cells that lacked gel contraction capacity (χ2 = 61; p =< 0.0001; n = 37). Thus, some HADF attained vasculogenic capacity and yet retained functional features of a fibroblast cell upon TNT mediated anti-miR-200b delivery…