Highlights
- •In vivo multimodal imaging identified early signs of transplant rejection
- •Systemic immunosuppression was necessary for long-term survival of xenotransplants
- •Human donor photoreceptors integrated and differentiated mostly into M/L cones
- •A degenerating retinal environment enhances structural synaptogenesis in PRPCs
Summary
Regenerative therapies aimed at replacing photoreceptors are a promising approach for the treatment of otherwise incurable causes of blindness. However, such therapies still face significant hurdles, including the need to improve subretinal delivery and long-term survival rate of transplanted cells, and promote sufficient integration into the host retina. Here, we successfully delivered in vitro-derived human photoreceptor precursor cells (PRPCs; also known as immature photoreceptors) to the subretinal space of seven normal and three rcd1/PDE6B mutant dogs with advanced inherited retinal degeneration. Notably, while these xenografts were rejected in dogs that were not immunosuppressed, transplants in most dogs receiving systemic immunosuppression survived up to 3–5 months postinjection. Moreover, differentiation of donor PRPCs into photoreceptors with synaptic pedicle-like structures that established contact with second-order neurons was enhanced in rcd1/PDE6B mutant dogs. Together, our findings set the stage for evaluating functional vision restoration following photoreceptor replacement in canine models of inherited retinal degeneration.
Introduction
Mammalian photoreceptors, the light-sensing outer retinal cells, lack self-regenerative capacity, and their degeneration in inherited retinal disease (IRD) is a major cause of blindness. However, while photoreceptors are lost in human IRDs and the dry form of age-related macular degeneration (AMD), the inner retinal structure is retained, even in advanced disease (
). As such, regenerative therapies aimed at replacing photoreceptors and establishing functional synapses with the remaining viable inner retinal neurons is a promising approach for restoring vision recovery in otherwise blind patients.
Human embryonic stem cells (hESCs) or induced pluripotent stem cell (iPSC)-derived photoreceptor precursor cells (PRPCs) that develop into retinal organoids (ROs) are a promising source for regenerative experimental therapies to restore photoreceptor function (
). Cell replacement studies in rodents have shown restoration of visual function using either ESC- or iPSC-PRPCs (
;
), and several clinical trials in humans are exploring the use of allogenic and autologous cell therapies for photoreceptor replacement (
). However, exogenous photoreceptor cell replacement therapies face critical challenges (
), including the need to (1) successfully deliver large numbers of cells subretinally to cover a large area, (2) ensure long-term photoreceptor survival rate by preventing immune rejection, and (3) promote sufficient integration into the host retina to recover visual function (
).
The dog offers unique advantages for advancing experimental retinal therapies (
). Multiple models of dogs with naturally occurring mutations in some of the genes responsible for several forms of human IRDs are available and exhibit similar biological, pathological, and functional deficits as those reported for the human disease (
). In addition, the size of the canine eye allows development and optimization of surgical and therapeutic approaches that can then be used in patients, and outcomes can be monitored using instruments commonly used in human clinics.
Here, we compared the ability of hPRPCs to survive and integrate in normal and in rcd1/PDE6B mutant dogs, an IRD model that undergoes early-onset progressive retinal degeneration primarily affecting rods. These mutant dogs undergo an acute phase of rods loss at 4–6 weeks of age that is followed by a progressive degeneration of rods and cones over the course of several months (
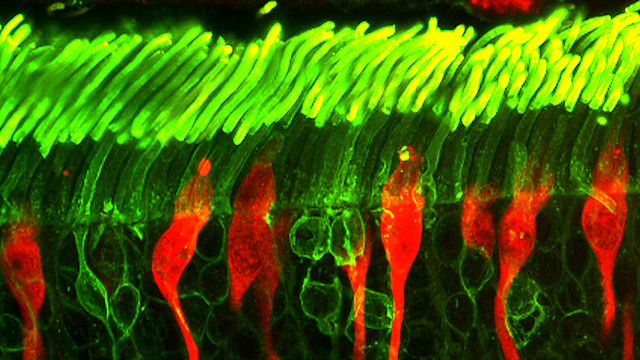
). Using a subretinal injector that was modified to accommodate hPRPC aggregates derived from ROs, we successfully delivered fluorescently tagged hPRPCs into the canine subretinal space (SRS) using a simple surgical procedure. A non-invasive multimodal imaging approach was used to assess cell viability and distribution and also enabled longitudinal analysis of individual cell clusters. A systemic immunosuppressive (IS) protocol of oral prednisolone, cyclosporine A (CsA), and mycophenolate mofetil (MMF) preserved-long term survival of hPRPC-derived cells. Donor cells in normal dogs receiving IS remained mostly in the SRS and attempted to create structural synapses but were largely unsuccessful, likely because of their inability to penetrate the intact outer limiting membrane (OLM). In contrast, the OLM of mutant dogs receiving IS was focally disrupted, and hPRPCs had extended axons and structural formations that resembled synaptic terminals, suggesting that disease-related retinal changes enhance the potential of hPRPCs to form synapses when properly integrated and polarized within the retina.
Results
Multimodal retinal imaging enables non-invasive monitoring of hESC-PRPCs injected into the SRS of normal and degenerated canine retinas
To accommodate the larger size of hPRPC aggregates obtained from ROs, we performed the subretinal delivery of donor cells using a custom-modified subretinal injector with a 31G or 33G cannula. With this approach, a prior vitrectomy was not required, and the cells could be delivered by bolus injection controlled by manual pressure. Human ESC-PRPCs derived from one of two photoreceptor reporter lines (either WA09 CRX-tdTomato or WA09 NRL-EGFP) (Phillips et al., 2018a, Phillips et al., 2018b) were successfully injected into the SRS of normal (12 eyes) and rcd1/PDE6B mutant (6 eyes) dogs without surgical complications. Details are listed in Table 1. In all dogs, some of the cell suspension was seen backflowing into the vitreous at the time of the bolus injection before the retinotomy was formed and the bleb began to expand. In non-vitrectomized eyes (n = 16), retinal blebs reattached within 24–48 h, while in the two vitrectomized eyes, 6–7 days were needed for full reattachment…