Abstract
Pluripotent stem cell (PSC)-derived human brain organoids enable the study of human brain development in vitro. Typically, the fate of PSCs is guided into subsequent specification steps through static medium switches. In vivo, morphogen gradients are critical for proper brain development and determine cell specification, and associated defects result in neurodevelopmental disorders. Here, we show that initiating neural induction in a temporal stepwise gradient guides the generation of brain organoids composed of a single, self-organized apical-out neuroepithelium, termed ENOs (expanded neuroepithelium organoids). This is at odds with standard brain organoid protocols in which multiple and independent neuroepithelium units (rosettes) are formed. We find that a prolonged, decreasing gradient of TGF-β signaling is a determining factor in ENO formation and allows for an extended phase of neuroepithelium expansion. In-depth characterization reveals that ENOs display improved cellular morphology and tissue architectural features that resemble in vivo human brain development, including expanded germinal zones. Consequently, cortical specification is enhanced in ENOs. ENOs constitute a platform to study the early events of human cortical development and allow interrogation of the complex relationship between tissue architecture and cellular states in shaping the developing human brain.
Introduction
Human brain development is unique in multiple aspects as compared to other mammals1,2,3, but its study has long been difficult due to the lack of suitable model systems. The development of three-dimensional (3D) in vitro human brain models, so-called organoids or spheroids, derived from pluripotent stem cells (PSCs) has emerged as a powerful tool to study brain development and associated diseases4,5,6,7. PSCs can be induced into neural fate and to reflect various regions of the brain using a regimen of small molecules and growth factors throughout the differentiation process, thereby attempting to mimic the signaling pathway activities throughout development8,9,10,11,12,13,14,15,16,17,18. Brain organoids have been amply shown critical to reveal important aspects of human brain development19,20,21,22,23,24,25. Nonetheless, some biological features are only limitedly recapitulated in brain organoids, such as the massive expansion of neural progenitors typical of the developing human brain5 and its overall organization26. In fact, within each brain organoid, a distinctive morphological feature is the uncontrolled and spontaneous development of multiple rosettes, representing individual neuroepithelium structures. This may cause within-and-between organoid heterogeneity and may affect reproducibility26. Additionally, this does not parallel in vivo brain organogenesis, where its development critically originates from a single neural tube. Indeed, recent efforts have aimed at mitigating the formation of multi-rosettes through the generation of single-rosette organoids, by employing either manual isolation of single rosette structures or using micro-patterning approaches27,28,29,30,31,32. Furthermore, the use of biomaterials or microdevices has also been exploited to guide the formation of enlarged or folded neuroepithelium structures in vitro33,34. Likewise, genetic approaches through PTEN knock-out can induce surface folding of brain organoids through enhanced expansion of the neural progenitors35.
Mammalian brain development is a highly regulated process shaped by strict time- and space-dependent morphogen gradients36,37,38, and associated dysregulation results in brain developmental defects. Recent efforts have indeed probed the use of genetic or synthetic morphogen gradient-based approaches to influence in vitro patterning outcomes, either for early germ layer patterning or for brain topography specification39,40,41. Creating a local source of BMP4 signaling using a microfluidic approach revealed important aspects of germ layer patterning39. In brain organoids, a sonic hedgehog gradient provided via an inducible genetic approach revealed its importance in patterning ventral forebrain fate40. Finally, a chip approach using morphogen-soaked beads directed the emergence of distinct dorso-ventral and anterior-posterior topography within brain organoids41. Here, we probed the effect of timed morphogen gradients during neural induction. We develop a straightforward approach based solely on a stepwise temporal medium gradient, which, strikingly, resulted in major morphological changes that ultimately generated well-specified cortical organoids displaying a self-organized single continuum of expanded neuroepithelium. We find that the presence of a prolonged gradient of TGF-β signaling is important in this process.
Results
A temporal neural induction gradient induces generation of expanded neuroepithelium organoids
The generation of brain organoids from PSCs relies on an initial neural induction, followed by expansion/differentiation and maturation phases42. These are typically induced by “all-at-once” switches to specific media that may vary in composition and timing depending on the specific protocol. Given that tight and time-controlled morphogen gradients underlie correct in vivo development, we reasoned that providing morphogen switches in a temporal and gradual (stepwise gradient) manner could influence brain organoid phenotypes.
After initial dissociation of feeder-free human embryonic stem cells (H1 hESCs), and reaggregation into embryoid bodies in stem cell medium, we employed dual SMAD inhibition11,13,43,44 for cortical neural induction (NI), and either switched to NI medium in a sudden- or stepwise manner (Fig. 1a and Supplementary Fig. 1a). We rigorously used the same number of initial cells for both protocols. In the stepwise, gradual NI protocol, cells are exposed to a prolonged and decreasing gradient of the stem cell medium, while concomitantly providing a stepwise, gradual increase in NI medium. Thereafter, medium was switched to expansion medium containing EGF and FGF2, and later to maturation medium from day 25 onwards (containing amongst others Matrigel). The fate during formation of the brain organoids under these different protocols was monitored over time. While prior as well as during NI the forming organoids were indistinguishable, approximately 12–14 days after protocol initiation, we observed a divergent morphological phenotype between organoids formed in the different conditions (Fig. 1b, c). Upon sudden NI, cortical organoids (COs) formed with a typical spherical shape with multiple rosettes visible under brightfield microscopy. Instead, under the stepwise gradual NI, organoids adopted a convoluted shape that became more pronounced with time (Fig. 1b and Supplementary Fig. 2a). At day 14, a clearly distinct lighter border with ridges and folds at the apex of the organoids was visible, which became more prolonged and pronounced at day 24 (Fig. 1b and Supplementary Fig. 2a), suggestive of expanded neuroepithelium structures.
We measured morphological features of the organoids under the two conditions during a 25-day time-course, which confirmed a strikingly reduced circularity under gradual NI, already evident at day 14, and progressively decreasing to 0.5 at day 25 (Fig. 1d). Furthermore, upon appearance of these folded structures, the gradual NI-generated organoids displayed an increased organoid perimeter (Fig. 1e). Of note, organoid areas were slightly, yet significantly, increased at day 20 and day 25 under stepwise NI (Supplementary Fig. 1g). Altogether, these morphological changes and parameters were consistent across multiple organoid batches formed under stepwise NI versus sudden NI (Supplementary Fig. 2a–c).
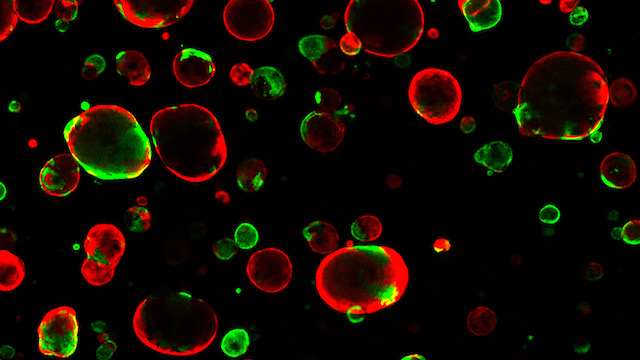
As an additional comparison, we generated organoids using a commercially available protocol for dorsal forebrain organoids (CommOs) (Supplementary Fig. 1b). As expected, also in this case the organoids formed with a typical spherical morphology (Supplementary Fig. 1c), and with morphological parameters analogous to the COs formed under the sudden NI protocol (Supplementary Fig. 1d, e). Clearly, the organoids generated under the gradual NI were morphologically very distinct (Fig. 1b and Supplementary Figs. 1c, 2a). We next analyzed the cellular organization of the different organoids by immunostaining using the neuroepithelium marker N-Cadherin (NCAD). At 16- and 24-days, COs as well as organoids generated using the commercial protocol, consisted of NCAD+ cells organized as a collection of multiple neural rosettes, displaying various shapes, some more elongated and some more circular and of different sizes (Fig. 1f, g and Supplementary Fig. 1f). Instead, the temporal gradient-generated organoids formed an elongated, continuous, radially organized, and often folded NCAD+ neuroepithelium, resembling a ventricular zone (VZ)-like structure (Fig. 1f, g and Supplementary Fig. 1f, Supplementary Videos 1, 2). NCAD+ cells of the neuroepithelium observed in these organoids were located on the outside of the organoid, suggesting an apical-out morphology (Fig. 1f, g and Supplementary Fig. 1f). We sometimes observed organoids in which, in addition to the extended neuroepithelium, a few rosette-like or spherical structures would additionally form (Supplementary Fig. 3a–c).
Despite the major cellular organization changes and the overall distinct organoid architecture, qPCR analysis confirmed the absence of off-lineage markers analogous to the conventional CO (sudden NI) protocol, while their neural identity was confirmed by robust NCAD and Nestin expression (Supplementary Figs. 1h, 2d). Given the appearance of these expanded neuroepithelium structures in contrast to the conventional rosette-like neuroepithelia, we named these organoids generated with the temporal stepwise NI gradient ENOs (Expanded Neuroepithelium Organoids). To test the robustness of ENO formation, we employed two additional widely used hESC lines (H9 and H14). Again, while sudden NI generated the typical, spherical rosette-containing COs (Supplementary Fig. 4a, b), the temporal stepwise NI gradient resulted into successful generation of expanded neuroepithelium structures (Fig. 1c, d and Supplementary Fig. 4a, b), across different batches using the H9 and H14 lines, similar to H1 ENOs (Fig. 1h). This was further corroborated by the notable differences in organoid circularity and the organoid perimeters in H9 and H14 ENOs as compared to the respective CO controls of each line, resembling our previous observations using H1 hESCs (Fig. 1d, e and Supplementary Fig. 4c–f). Finally, NCAD staining of H9 and H14 ENOs revealed similar cellular organization and tissue architecture as observed for H1 ENOs (Supplementary Figs. 1i, 3a–c).
TGF-β signaling gradient plays a major role in determining ENO formation
To understand which signaling pathway could be involved in the formation of ENOs, we carefully considered the composition of both the stem cell medium, which is gradually reduced during NI in a stepwise fashion, and of the NI medium which is instead gradually increased (Fig. 1a). FGF2 and TGF-β are the main morphogens present in the stem cell medium and are both needed for the maintenance of an undifferentiated embryonic stem cell state45,46. Our NI medium is instead based on a classical dual SMAD inhibition approach43, in which the use of the TGF-β inhibitor (SB-431542, SB43) and the BMP inhibitor (dorsomorphin, DSM)43,47,48 are provided during early stages of organoid formation to specify dorsal fate11,12,13,44. Given the concomitant decreasing gradient of TGF-β as well as the increasing TGF-β inhibitor concentration during neural induction in ENOs, we evaluated whether such controlled TGF-β gradient was responsible for the observed drastic phenotype of ENOs. We, therefore, generated organoids under different levels of TGF-β signaling gradient modulation during NI from starkest-to-lowest inhibition over time (Fig. 2a, b): (1) the conventional COs, in which TGF-β is suddenly removed and replaced by its inhibitor SB43, therefore driving the most abrupt switch-off of TGF-β signaling; (2) a “full SB43” protocol, in which TGF-β in the stem cell medium is still decreased in a step-wise fashion, but the full inhibition by SB43 is added “all-at-once” on the first day of the neural induction, therefore still providing a more abrupt inhibition of TGF-β signaling; (3) the ENOs in which there is a period of concomitant decreasing gradient of TGF-β as well as an increasing gradient of SB43 concentration; and (4) a “no SB43” protocol, in which the concentration of TGF-β is decreased step-wise but is not counteracted by SB43, implying that its presence persists the longest in culture and therefore has the longest active signaling gradient. Considering day 0 to have the maximum presence of TGF-β (100%), all tested protocols start with the same concentration (Fig. 2a)….