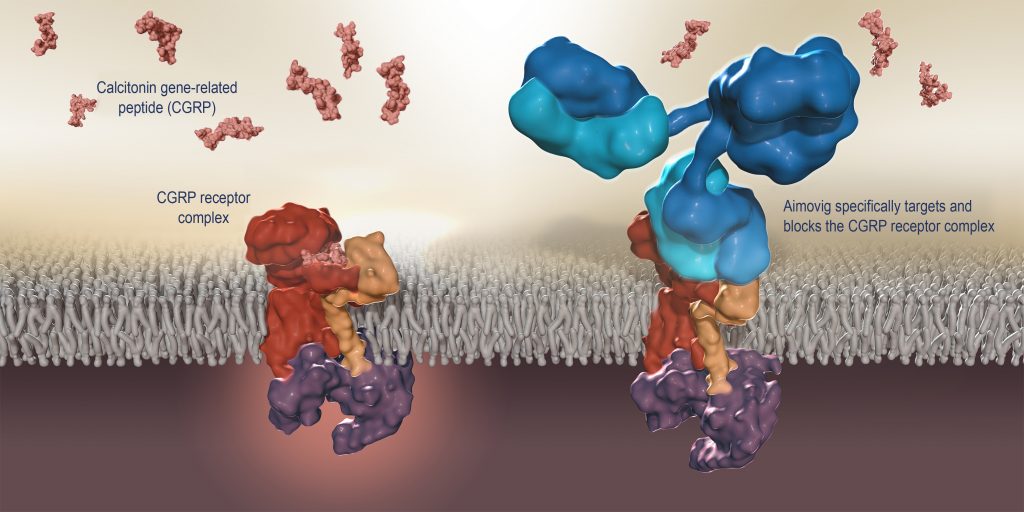
Amgen and Novartis are set to commercially launch the first calcitonin gene-related peptide (CGRP) receptor inhibitor in the U.S. next week following FDA approval yesterday of the companies’ co-marketed migraine prevention treatment Aimovig™ (erenumab).
Aimovig will be the first-and-only monoclonal antibody specifically designed to prevent migraines by blocking the CGRP receptor believed to play a causal role in migraine pathophysiology. Aimovig has been projected by Evaluate Pharma to generate about $475 million in sales by 2022.
Aimovig is expected to be available to patients in the U.S. within a week, Amgen and Novartis said.
“We look forward to working closely with Amgen in the U.S. to bring this treatment to physicians and their patients, who could now gain days of their lives back each month,” Paul Hudson, CEO of Novartis Pharmaceuticals, said in a statement.
In Europe, the European Medicines Agency (EMA) is reviewing a Marketing Authorization Application (MAA) for the drug, with Novartis stating that it expects European Commission approval “in the coming months.”
The FDA gave its approval a day ahead of its May 18 target decision date under the Prescription Drug User Fee Act (PDUFA). The agency accepted the Biologics License Application (BLA) of Amgen and Novartis for Aimovig in July 2017.
“We Need New Treatments”
“Aimovig provides patients with a novel option for reducing the number of days with migraine,” Eric Bastings, M.D., deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research,” said in a separate statement issued yesterday by the agency. “We need new treatments for this painful and often debilitating condition.”
Amgen and Novartis said the efficacy, tolerability, and safety of Aimovig was assessed in clinical studies with more than 3000 participants—including the Phase IIIb LIBERTY study (NCT03096834) and an ongoing open-label extension of up to five years in duration.
In LIBERTY, which assessed the drug in patients with episodic migraine who have failed two to four prior treatments, patients who took Aimovig 140 mg had nearly three-fold higher odds of having their migraine days cut by half or more compared to placebo. The most common adverse reactions in the clinical studies were injection site reactions and constipation, Amgen and Novartis said.
Aimovig 70 mg is self-administered once-monthly via Amgen’s SureClick® autoinjector device, which does not require a loading dose. Some patients may benefit from a dosage of 140 mg once monthly, Amgen and Novartis said.
The U.S. list price of Aimovig is $575 for once-monthly 70-mg or 140-mg single-use prefilled SureClick autoinjectors, or $6900 annually. The companies said they will assist patients in navigating insurance coverage and accessing the drug through the Aimovig AllyTM product support program.