Plaques of amyloid-β peptides in certain parts of the brain are a telltale sign of Alzheimer’s disease–associated neurodegeneration. When not in pathological aggregates, these peptides—found in healthy brains at low levels—are commonly considered inefficiently cleared catabolic byproducts. Now, researchers at Harvard and Massachusetts General Hospital (MGH) and their colleagues have shown that amyloid-β can protect against yeast and bacterial infections in two animal models, as well as in cultured human cells. Amyloid-β was able to bind carbohydrates on the surfaces of microbes, preventing the invaders from binding host cells. The team’s findings, published today (May 25) in Science Translational Medicine, suggest that amyloid-β may function similarly to antimicrobial peptides of the innate immune system.
“This is quite good and convincing work that confirms host defense activity of amyloid-β against fungal and bacterial infections in animal models, and begins to unravel the mechanisms of antimicrobial activity of the protein,” said Kevan Hartshorn who studies innate immunity at the Boston University School of Medicine and was not involved in the study.
“Amyloid-β is overdue for an update,” said Douglas Ethell, a neuroscientist at Western University of Health Sciences in California who was not involved in the work. “For too long it’s been viewed as a useless byproduct that wreaks havoc on the human brain. This paper adds to a growing body of evidence that amyloid-β serves important physiological roles that we are only now beginning to understand.”
Rudolph Tanzi and Robert Moir, who study neurodegeneration at Harvard MGH, sought to test whether amyloid-β might protect mice, Caenorhabditis elegans nematodes, and human cells in culture from invasion by various microbes.
Young mice that expressed high levels of human amyloid-β (but did not have pathological plaques) infected in the brain with Salmonella typhimurium were more likely to survive the infection compared to wild-type mice that did not express the peptide, Tanzi, Moir, and their colleagues found.
“Overnight, the injected bacteria caused plaque deposits with a single bacterium in the middle,” said Tanzi. “We think about the slow production of amyloid plaques over decades and in this mouse model, a bacterial infection could cause full-blown plaques overnight.”
C. elegans expressing a modified form of human amyloid-β survived three or four more days following infection in the gut with Candida albicans, compared to wild-type worms that did not express the peptide.
In vitro, transforming human brain neuroglioma or Chinese hamster ovary cells with an amyloid-β isoform resulted in better survival following a yeast infection. Fewer fungal cells were able to adhere to the transformed cells compared to control cells that did not express the peptide, the researchers found.
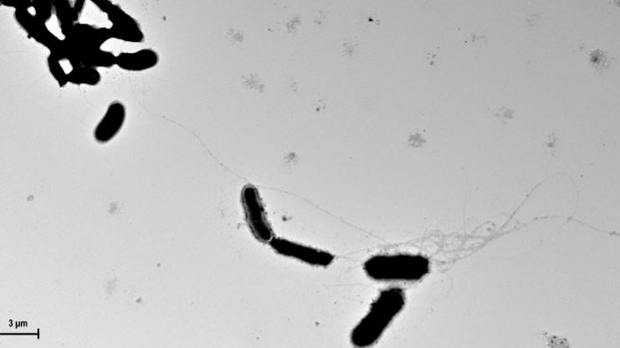
Found throughout the body, human antimicrobial peptides form fibrils and oligomers, capturing microbes within these structures. Similar to antimicrobial peptides, the team showed, in culture, fibrils of amyloid-β were able to entangle yeast cells, forming clumps.
“Based on our work, it’s possible that amyloid works in a similar way, quickly seeded in the brain in response to a microbial pathogen,” Tanzi told The Scientist.
Using microscopy, Tanzi, Moir, and their colleagues observed that amyloid-β formed fibrils and trapped C. albicans cells in the guts of infected worms. In mice, S. typhimurium cells were found entangled within amyloid-β deposits; in noninfected mice, the peptides did not form fibrils.
With its report, the Harvard/MGH-led team is the first to show antimicrobial activity of amyloid-β in animals. Moir, Tanzi, and their colleagues had previously demonstrated that both a synthetic form of amyloid beta and amyloid peptides from the brains of Alzheimer’s patients could prevent the growth of C. albicans in a similar way to the antimicrobial activity of a human antimicrobial peptide called LL-37. “Whereas amyloid-β is viewed as a piece of junk, LL-37 is [considered] a key antimicrobial peptide important for immunity,” said Moir.
The team will next look for pathogens in the brains of people with Alzheimer’s disease.
“Since the properties that make the peptide problematic are the very properties that make it effective in host defense,” said Hartshorn, “the next question is whether we can find a balance to both prevent plaque formation in the brain and not damage the immune activity of amyloid-β.”