The transcription factor Prdm16 functions as a potent suppressor of transforming growth factor-beta (TGF-β) signaling, whose inactivation is deemed essential to the progression of pancreatic ductal adenocarcinoma (PDAC). Using the KrasG12D-based mouse model of human PDAC, we surprisingly found that ablating Prdm16 did not block but instead accelerated PDAC formation and progression, suggesting that Prdm16 might function as a tumor suppressor in this malignancy. Subsequent genetic experiments showed that ablating Prdm16 along with Smad4 resulted in a shift from a well-differentiated and confined neoplasm to a highly aggressive and metastatic disease, which was associated with a striking deviation in the trajectory of the premalignant lesions. Mechanistically, we found that Smad4 interacted with and recruited Prdm16 to repress its own expression, therefore pinpointing a model in which Prdm16 functions downstream of Smad4 to constrain the PDAC malignant phenotype. Collectively, these findings unveil an unprecedented antagonistic interaction between the tumor suppressors Smad4 and Prdm16 that functions to restrict PDAC progression and metastasis.
Introduction
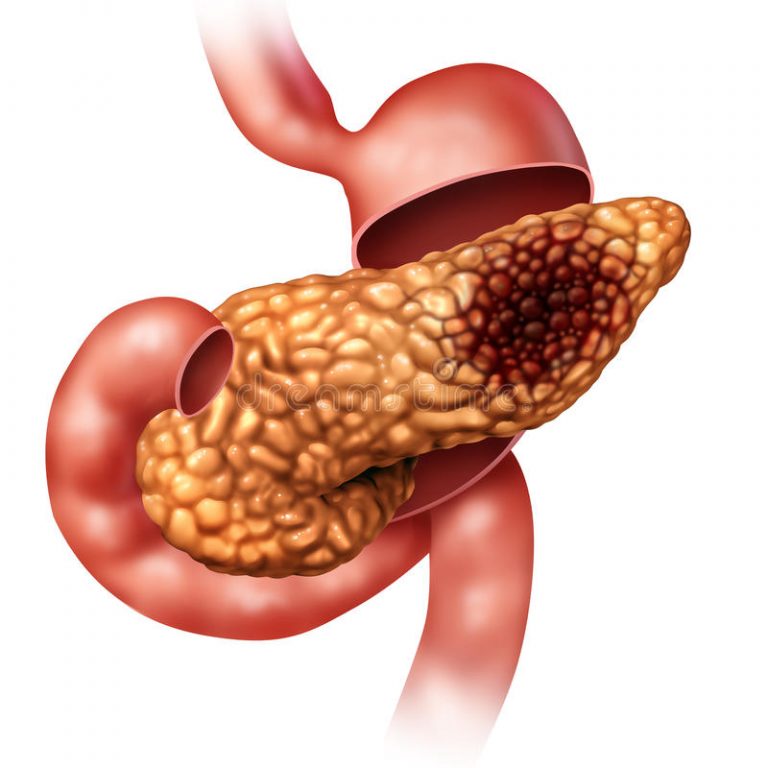
Pancreatic ductal adenocarcinoma (PDAC) is the most aggressive type of pancreatic cancer, currently ranked as the fourth leading cause of cancer-related deaths in the United States (Connor and Gallinger, 2021; Hidalgo, 2010). Most of PDAC patients present with both locally invasive tumors and widespread metastasis, thus rendering ineffective the resection of the primary tumor as well as the applicability of the dismal therapeutic options available (Hidalgo, 2010; Stathis and Moore, 2010). Consequently, the outcome of PDAC patients remains extremely poor, with an overall 5-yr survival rate of less than 11%.
PDAC tumors emerge through three types of distinct precursor lesions called pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasia (IPMN), and mucinous cystic neoplasia (MCN), respectively (Connor and Gallinger, 2021; Yonezawa et al., 2008). These early-stage lesions harbor various genetic alterations, the earliest and most pervasive of which are activating mutations in KRAS, occurring in ∼90% of PDAC tumors (Hayashi et al., 2021). The current model posits that mutational activation of KRAS represents an essential initiating event, whereas subsequent accumulation of inactivating mutations in the tumor suppressor genes p16INK4a, SMAD4, and TP53 is necessary for PDAC to progress and metastasize (Hayashi et al., 2021; Iacobuzio-Donahue, 2012). Significant efforts have been made over the past two decades to create genetically engineered mouse models (GEMMs) that faithfully recapitulate the prominent features of human PDAC. For instance, pancreas-specific expression of KrasG12D in mice is sufficient to initiate PanINs, which occasionally progress into invasive PDAC following a long latency period, supporting the general notion that oncogenic activation of KRAS represents the main initiating genetic event in PDAC (Buscail et al., 2020; Hingorani et al., 2005; Tuveson et al., 2004; Westphalen and Olive, 2012). Concomitant expression of KrasG12D and deletion of any of the three cardinal tumor suppressors, e.g., Trp53, p16Ink4a, Smad4, accelerate PDAC progression, though the nature and final outcome of the tumors might differ. Indeed, while mice with the combined expression of KrasG12D and deletion of Trp53 (KPC) or p16Ink4a (KIC) develop PanINs that progress very rapidly to highly aggressive and metastatic PDAC, mice with the combined expression of KrasG12D and deletion of Smad4 (KSC) develop mostly IPMNs, which also progress to invasive PDAC, but the terminal disease develops with a much slower onset and manifests an attenuated metastatic phenotype (Bardeesy et al., 2006a; Bardeesy et al., 2006b; Hingorani et al., 2005; Izeradjene et al., 2007). Other examples of PDAC GEMMs include KTβC mice, which harbor KrasG12D and deletion of the transforming growth factor-beta (TGF-β) type II receptor (TβRII) gene, the latter being inactivated by mutations or deletions in 4% of PDAC (Iacobuzio-Donahue, 2012; Ijichi et al., 2006).
TGF-β signaling regulates a wide array of biological processes vital for normal cell growth, function, and homeostasis (David and Massagué, 2018; Massagué, 2008). TGF-β initiates signaling by inducing the assembly of a receptor complex composed of two types of transmembrane serine/threonine kinases called TβRI and TβRII. In that complex, the constitutive kinase of TβRII phosphorylates and activates the kinase activity of TβRI, which then propagates the signal to the nucleus through phosphorylation of Smad2 and Smad3 (David and Massagué, 2018; Feng and Derynck, 2005; Massagué et al., 2005). Once phosphorylated, Smad2 or Smad3 associates with Smad4, and the two complexes accumulate in the nucleus to regulate the expression of TGF-β target genes through cooperative interactions with transcriptional cofactors or corepressors (David and Massagué, 2018; Feng and Derynck, 2005; Massagué, 2008; Massagué et al., 2005).
Because of the widespread roles of TGF-β signaling in cellular functions, there must be multiple levels of positive and negative regulations to fine-tune initiation, magnitude, or termination of the response depending on the cell type or physiological context. One example of the mechanisms that limit TGF-β signaling involves the transcription factor PR domain containing 16 (Prdm16). Upon accumulation in the nucleus, the Smad3/Smad4 complex associates with the general transcriptional co-activators CBP and p300 to activate transcription of TGF-β target genes (David and Massagué, 2018; Feng and Derynck, 2005; Massagué, 2008; Massagué et al., 2005). Conversely, the Smad complex can also associate with Prdm16 and its partner c-Ski, which leads to the recruitment of general transcriptional corepressor complexes containing histone deacetylases and concomitant displacement of CBP and p300, thereby resulting in transcriptional repression (Takahata et al., 2009).
In addition to its function as a suppressor of TGF-β signaling, Prdm16 has been shown to play key roles in a number of biological processes, including differentiation of brown fat and specification of hematopoietic and neuronal stem cell fate (Chi and Cohen, 2016; Seale et al., 2007; Shimada et al., 2017). Prdm16 possesses a methyltransferase activity that catalyzes the methylation of Lysine-9 on histone-3 (H3K9), a mark associated with heterochromatin formation and gene expression (Jambhekar et al., 2019; Pinheiro et al., 2012). Recently, Prdm16 loss-of-function has been shown to play an instrumental role in leukemia driven by the MLL fusion oncoprotein (Zhou et al., 2016). Because the MLL gene encodes a histone-3 Lysine-4 (H3K4) methyltransferase that is critical in promoting gene expression during hematopoiesis (Xue et al., 2019), it has been postulated that Prdm16 might suppress leukemia pathogenesis owing to its ability to drive heterochromatin formation (Pinheiro et al., 2012; Zhou et al., 2016). At present, whether Prdm16 has any role in cancer pathogenesis and progression that is linked to its function in TGF-β signaling is still unknown. Here, we combined several orthogonal approaches and GEMMs to demonstrate that Prdm16 functions downstream of Smad4 to suppress PDAC progression and metastasis. As such, our findings unveil a previously uncharacterized mechanism that orchestrates Prdm16 tumor-suppressive function, and further shed new insights into the molecular etiology of PDAC, a fatal disease for which no effective therapeutics are currently available.
Results
Transient expression of Prdm16 during PDAC progression
To explore the possible involvement of Prdm16 in PDAC, we conducted Kaplan-Meier analysis using The Cancer Genome Atlas (TCGA) dataset. As shown in Fig. 1 A, low PRDM16 expression is associated with poor survival, providing an initial hint that Prdm16 might function as a tumor suppressor in PDAC. To substantiate this finding, we analyzed Prdm16 expression by immunohistochemistry (IHC) using large human tissue microarrays (TMAs) comprising samples with tumor lesions at various stages (e.g., PanIN1, PanIN2, PanIN3, PDAC) and normal tissues. Using a highly specific antibody to Prdm16 (see Fig. S2 A), we detected Prdm16 expression in both cancerous lesions and stromal areas (Fig. 1 B). Interestingly, Prdm16 expression appeared to fluctuate significantly during PDAC progression, commencing with a relatively low level in normal tissue, then rising in early PanINs, and finally declining to the background level in invasive PDAC (Fig. 1 B). Although this finding fits well with the notion that Prdm16 expression might be downregulated because of the accumulation of late genetic or epigenetic alterations, it did not shed light on the mechanisms leading to its transient expression during PDAC progression. To address this issue rigorously, we sought to utilize GEMMs that faithfully recapitulate the human PDAC in a uniform genetic background (Bardeesy et al., 2006a; Bardeesy et al., 2006b; Hingorani et al., 2005; Izeradjene et al., 2007; Tuveson et al., 2004). We initially utilized mice with pancreas-specific expression of KrasG12D alone (KC) and detected transient expression of the Prdm16 protein during PDAC progression, similar to what was observed in human PDAC, being relatively high in PanINs and very modest to low in normal tissue and invasive PDAC (Fig. 1 C). Confirmation of this result was obtained by comparative qRT-PCR experiments using cohorts of KC mice at the age of 3 mo when they experience mostly PanINs and 10 mo when they display visible signs of terminal PDAC (Fig. 1 D; Parajuli et al., 2020; Parajuli et al., 2019). To understand this phenomenon more deeply, we generated mice with KrasG12D together with deletion of Trp53 (KPC), p16Ink4a (KIC), or Smad4 (KSC). Noteworthy, we found that KSC mice had a longer survival rate than KIC and KPC mice, while the two latter had almost similar survival (Fig. S1 A). With regard to Prdm16 expression, we found that KIC and KPC mice behaved similarly to KC mice (Fig. S1, B and C), suggesting that transient expression of Prdm16 might take place even under the presence of the most common and aggressive genetic alterations that facilitate PDAC progression (Hayashi et al., 2021; Iacobuzio-Donahue, 2012). But most appealing was the fact that Prdm16 expression in KSC mice did not follow this transient pattern of Prdm16 expression, increasing markedly in IPMN lesions but thereafter remaining constant in PDAC lesions (Fig. 1 E), suggesting that Smad4 might influence Prdm16 expression during the progression from IPMN to full-blown PDAC. Co-immunofluorescence assays using anti-Prdm16 antibody together with antibodies to E-cadherin (epithelial marker) or vimentin (mesenchymal marker) showed that Prdm16 expression remained very high in E-cadherin + cells as compared to vimentin + cells (Fig. S1 D). Consistent with these findings, we found that patients with low expression of Prdm16 had the worst survival if they carry SMAD4 mutations (Fig. S1 E). Moreover, interrogating the TCGA dataset revealed that samples with deleterious genetic alterations in SMAD4 display higher expression of PRDM16 as compared to samples with wild-type SMAD4 (Fig. 1 F). As such, these data hint at the existence of an antagonistic association between Smad4 and Prdm16 during PDAC progression; we will return to this notion later.
Prdm16 accelerates KrasG12D-driven PDAC
The aforementioned data prompted us to investigate whether Prdm16 could contribute to PDAC initiation, progression, or both. To do so, we generated mice with pancreas-specific deletion of Prdm16 (Prdm16KO) by crossing mice bearing a floxed allele of Prdm16 with Pdx1-Cre mice, which express Cre recombinase in all pancreatic progenitor cells that give rise to ductal, acinar, and islets compartments very early (E8.5) during development (Gu et al., 2003). Prdm16KO mice were born with the normal Mendelian frequency, develop normally without any signs of anatomic abnormalities, and were fertile. Effective deletion of Prdm16 in the pancreatic epithelium was confirmed by RT-PCR and IHC (Fig. 2 A and Fig. S2 A). To investigate whether Prdm16 deficiency could affect pancreas histology or function, we conducted a comprehensive analysis of pancreatic sections either by hematoxylin and eosin (H&E) staining, IHC or immunofluorescence (IF) encompassing all major tissue compartments, including duct (cytokeratin 19, CK19), acini (amylase), stroma (α-SMA), and islet (insulin, glucagon, chromogranin-A). We were not able to detect any noticeable changes in all three compartments irrespective of the age of mice analyzed (Fig. 2, B–D; and Fig. S2, B and C). Congruently, there was also no difference in fasting blood glucose between wild-type and Prdm16KO mice (Fig. S2 D). Thus, inactivation of Prdm16 throughout embryonic development and postnatal life was insufficient to perturb pancreas homeostasis or drive sporadic pancreatic cancers.
Next, we sought to investigate whether Prdm16 could influence PDAC progression initiated through activation of Kras signaling. The salient genetic features of PDAC originate with the near-ubiquitous gain of function mutations in KRAS in their incipient stage. However, progression to invasive PDAC in KrasG12D-bearing mice has proved to be either a protracted or unachieved process, as a small fraction of mice succumb directly to PDAC following a very long latency period (Hingorani et al., 2005; Parajuli et al., 2020; Parajuli et al., 2019; Tuveson et al., 2004). It is widely believed that the acquisition of secondary mutations in certain tumor suppressors can endow transformed cells with the growth advantage needed for disease progression. For instance, combining KrasG12D with deletion of Smad4 or TβRII has been shown to accelerate the progression of PDAC, which was thought to be conferred through disruption of TGF-β cytostatic signaling (Bardeesy et al., 2006b; Ijichi et al., 2006; Izeradjene et al., 2007). Given its role as an inhibitor of Smad signaling, we surmised that Prdm16 inactivation might suppress PDAC development and/or progression owing to the de-repression of TGF-β/Smad signaling. To probe this possibility, we generated mice harboring KrasG12D alone (KC) or in combination with conditional deletion of both alleles of Prdm16 (KPrC) and conducted comparative studies to analyze their PDAC phenotypes. Consistent with previous studies (Parajuli et al., 2020; Parajuli et al., 2019; Tuveson et al., 2004), KC mice maintained uniformly good health until around the age of 20 wk, and thereafter a fraction of mice became suddenly morbid and succumbed within days to an aggressive PDAC. Contrary to our prediction, combining Prdm16 deletion with KrasG12D instead resulted in a marked acceleration of PDAC. Kaplan-Meyer analysis showed a significant decrease in the median survival of KPrC mice as compared to KC mice (Fig. 3 A). During an observation period of 6 mo, 70% of KPrC mice succumbed to PDAC, whereas more than 76% of KC mice survived and remained free of invasive PDAC (Fig. 3 A). To confirm this finding, we conducted histopathological analysis with pancreatic sections from KPrC and KC mice of the same age that showed either relatively healthy appearance or signs of morbidity characteristic of invasive PDAC at the time of necropsy. At early stages of tumorigenesis, KPrC mice displayed a significant increase in PanIN lesions compared to KC mice, as assessed by H&E and IHC using anti-CK19 antibody (Fig. 3 B). A similar conclusion could be drawn while analyzing another ductal marker, MUC5AC, either by IHC or Alcian blue staining (Fig. S3). KPrC and KC mice with invasive PDAC also showed clear difference in both tumor architecture and reactivity to the anti-CK19 and anti-Mu5AC antibodies as well as to Alcian blue (Fig. 3 B and Fig. S3). Moreover, IHC analysis using anti-α-SMA antibody showed more extensive stroma both within and outside PDAC lesions in KPrC mice relative to KC mice (Fig. S3). An automatic-guided quantification confirmed the increase in the surface areas of PanIN and PDAC lesions in KPrC mice as compared to KC mice (Fig. 3 B). Thus, Prdm16 inactivation appeared to accelerate PDAC once it has been initiated through activation of oncogenic KrasG12D signaling.
Requirement of Prdm16 for IPMN-to-PDAC progression
Given the inverse association between Smad4 and Prdm16 that we noticed earlier during PDAC progression (Fig. 1, E and F), we sought to extend our genetic approaches to explore whether Prdm16 could play a role, if any, in PDAC that depends on its function in TGF-β/Smad signaling. Accordingly, we generated mice with the combined deletion of Prdm16 and Smad4 in a KrasG12D background (KSPrC). KPrC, KSC, KC, and wild-type mice were used as controls. KSPrC mice were born with Mendelian frequencies, and no phenotypic differences between KSPrC and KSC mice were observed. Strikingly, however, the vast majority of KSPrC mice became stunted and morbid in appearance within 2 to 3 wk of weaning, and only 25% of them survived beyond 3 mo (Fig. 4 A). During this period, most of KPrC and KSC mice (84 and 90%, respectively) did not develop or succumb to PDAC. To elucidate the mechanism causing the acceleration of PDAC in KSPrC mice, we conducted histopathological analyses to study different stages of PDAC from the premalignant lesions to invasive adenocarcinomas. We found that KSC pancreas displayed predominantly macroscopic cystic lesions reminiscent of IPMN, as evidenced by the overall architecture as well as the high reactivity to the anti-Muc5AC and anti-CK19 antibodies as well as Alcian blue (Fig. 4 B and Fig. S4 A). In contrast, KSPrC pancreas displayed none to very few IPMN lesions (Fig. 4 B and Fig. S4 A). At the stage of full PDAC, KSPrC tumors were poorly differentiated adenocarcinomas, characterized by loss of the epithelial marker E-cadherin and acquisition of the mesenchymal marker vimentin (Fig. S3 B), which could be due either to increased accumulation of cancer associated fibroblasts or epithelial to mesenchymal transition (EMT), the latter being a general hallmark of metastasis (Pei et al., 2019). In marked contrast, KSC tumors were well differentiated with little or no change in E-cadherin or vimentin expression (Fig. S4 B), in line with previous studies that KSC mice are resistant to metastasis (Bardeesy et al., 2006b; Izeradjene et al., 2007; Whittle et al., 2015). As concomitant inactivation of Prdm16 appeared to shift the evolution of the IPMN-to-PDAC progression sequence toward the PanIN-to-PDAC progression sequence, it is tempting to speculate that Prdm16 might function at the stage of early preneoplastic lesions to influence PDAC development and progression. In support of this notion, deleting PRDM16 in two fully-transformed human PDAC cell lines (i.e., PANC-1, sufficient for SMAD4 and BxPC-3, deficient for SMAD4) did not affect their proliferative or invasive behaviors, as gauged by a combination of in vivo and in vitro assays (Fig. S4, C–F).
The poor prognosis for human PDAC is mainly due to quasi-inevitable metastasis affecting the liver and lung at the time of diagnosis (Connor and Gallinger, 2021; Hidalgo, 2010). Due to the severity of PDAC in KSPrC mice, we wondered whether concomitant deletion of Prdm16 could confer metastatic ability to the otherwise non-metastasizing PDAC tumors that typically develop in KSC mice (Bardeesy et al., 2006b; Izeradjene et al., 2007; Whittle et al., 2015). Indeed, we consistently observed the presence of metastatic lesions in the lung in all KSPrC mice that developed invasive tumors but survived until necropsy (Fig. 4 C). In contrast, no metastatic lesions were detected in KSC mice even with terminal PDAC (Fig. 4 C), as previously described (Bardeesy et al., 2006b; Izeradjene et al., 2007; Whittle et al., 2015). Confirmation of these results was obtained by IHC using an antibody to the PDAC marker CK19 (Fig. 4 C). Collectively, these data demonstrate that concomitant inactivation of Prdm16 was sufficient to confer metastatic properties on non-metastatic KSC tumors, a phenomenon that is associated with a shift from the IPMN-to-PDAC phenotype to the PanIN-to-PDAC phenotype.
Repression of Prdm16 expression by Smad4
To investigate the molecular mechanisms by which Prdm16 controls PDAC progression and metastasis in the context of a Smad4 null background, we took advantage of our earlier IHC analysis showing that Smad4 deficiency in KSC mice was associated with a persistent de-repression of Prdm16 during the progression from IPMN to PDAC (Fig. 1 E). We surmised that Smad4 might function either directly or indirectly to repress Prdm16 expression, which in turn impacts the progression trajectory of PDAC. We initially conducted qRT-PCR experiments using KSC mice, and found that the increase in Prdm16 expression was mediated at least via gene expression (Fig. 5 A). Because Smad4 functions as an essential component of TGF-β signaling (David and Massagué, 2018; Feng and Derynck, 2005; Massagué, 2008), we next wondered whether activation of TGF-β signaling could repress Prdm16 expression, as does Smad4. To our surprise, treating mouse PDAC cells KPC1 or human PDAC cells PANC-1 with TGF-β1 instead elicited a marked increase in Prdm16 expression (Fig. 5, B–D). As a specificity control, TGF-β1 treatment failed to induce Prdm16 expression in the human PDAC cell line MIA-PaCa-2 (Fig. 5 E), which lacks a functional TGF-β receptor (Freeman et al., 1995). To determine whether the effect of TGF-β1 is mediated via Smad4, we conducted comparative experiments using PANC-1 cells deleted of SMAD4 by CRISPR/CAS9. We found that ablating SMAD4 resulted in a marked increase in the steady-state expression of Prdm16 mRNA and protein (Fig. 5 F, see also Fig. 6 F), confirming the ability of endogenous Smad4 to repress PRDM16 expression in human cells. Intriguingly, challenging cells with TGF-β1 did not further increase Prdm16 expression in cells deleted of SMAD4 as compared to cells expressing the control gRNA (Fig. 5 F; see also Fig. 6 F), implying that SMAD4 inactivation is sufficient to mimic the effects of TGF-β1 stimulation.
Previous studies have shown that Smad proteins can stimulate or repress expression of TGF-β responsive genes through direct binding to their promoter (David and Massagué, 2018; Feng and Derynck, 2005; Massagué, 2008). In addition, a substantial fraction of Smad4 has been shown to localize in the nucleus in the absence TGF-β stimulation, but the physiopathological significance of this phenomenon remains unknown (Pierreux et al., 2000). Because SMAD4 ablation in PANC-1 cells was sufficient to recapitulate the stimulatory effects of TGF-β signaling on PRDM16 expression, we initially reasoned that Smad4 might bind to and repress the PRDM16 promoter at steady state, and that TGF-β signaling activation might displace Smad4 from the PRDM16 promoter. Accordingly, we conducted ChIP experiments, focusing on Smad conserved binding elements (SBE) within the PRDM16 promoter that we identified through an in-silico analysis. Using chromatin from PANC-1 cells, we detected a strong binding of Smad4 to the PRDM16 promoter at steady state (Fig. 6 A)….