Highlights
- •FOXG1 syndrome neural progenitor cells show slower G1 phase exit and proliferation
- •FOXG1 syndrome neural progenitor cells have increased frequency of primary cilia
- •Engineered FOXG1 dose correlates with proliferation and frequency of primary cilia
Summary
Heterozygous loss-of-function mutations in Forkhead box G1 (FOXG1), a uniquely brain-expressed gene, cause microcephaly, seizures, and severe intellectual disability, whereas increased FOXG1 expression is frequently observed in glioblastoma. To investigate the role of FOXG1 in forebrain cell proliferation, we modeled FOXG1 syndrome using cells from three clinically diagnosed cases with two sex-matched healthy parents and one unrelated sex-matched control. Cells with heterozygous FOXG1 loss showed significant reduction in cell proliferation, increased ratio of cells in G0/G1 stage of the cell cycle, and increased frequency of primary cilia. Engineered loss of FOXG1 recapitulated this effect, while isogenic repair of a patient mutation reverted output markers to wild type. An engineered inducible FOXG1 cell line derived from a FOXG1 syndrome case demonstrated that FOXG1 dose-dependently affects all cell proliferation outputs measured. These findings provide strong support for the critical importance of FOXG1 levels in controlling human brain cell growth in health and disease.
Introduction
The mammalian brain is a remarkable organ that must undergo several steps in development to ensure proper formation of specific brain structures. One unique structure of the brain is called the cerebral cortex, and it is derived from neural progenitor cells (NPCs) (Foley et al., 2000; Nieuwkoop, 1947; Rakic, 2006). NPCs proliferate to a precise number, differentiate, and migrate to become cerebral cortical cells. Cellular expansion prior to cell differentiation is tightly regulated (Caviness et al., 1995; Rakic and Caviness, 1995), but how NPCs know when to stop proliferating and begin differentiating is not well understood. This remains a fundamental question despite extensive work to reveal important parts of this pathway (Egger et al., 2011; Homem et al., 2015). The factors that control when NPCs proliferate or differentiate (Ernst, 2016) are fundamental to understanding many neurodevelopmental diseases in humans.Microcephaly is a disorder of development affecting about 7 in 10,000 live births (Hanzlik and Gigante, 2017). It is defined as a head circumference less than 2 standard deviations below the mean for gender and age and can be present from birth (termed “primary” microcephaly) or post-natally. Glioblastoma multiforme (GBM) is a severe form of brain tumor that has a median survival of 15 months from diagnosis and an incidence rate of 3 per 100,000 people per year (Cloughesy et al., 2014). A core feature of both primary microcephaly and GBM is that they likely depend on the activity of NPCs. NPCs must expand significantly as the brain develops to form the appropriate number of cells, whereas too little expansion can lead to primary microcephaly. In GBM, hallmark genes of NPCs are reactivated to drive tumor cell growth (Lathia et al., 2015). An understanding of the critical regulators of NPC proliferation is thus essential for understanding both primary microcephaly and GBM.FOXG1 is a member of the FOX superfamily, characterized by the amino acid forkhead domain, that associates with DNA to affect neurodevelopmental programming (Golson and Kaestner, 2016). It is uniquely brain expressed (Murphy et al., 1994) and there is significant evidence supporting the role of FOXG1 in expanding the NPC pool from human and mouse studies. Human FOXG1 syndrome (OMIM: 164874), in which one copy of FOXG1 is mutated, leading to loss of function, is a recognized microcephaly syndrome (Ariani et al., 2008). Foxg1 knockout in mice leads to an absent or extremely stunted telencephalon (Hanashima et al., 2004; Xuan et al., 1995). Without Foxg1 or with reduced dosage, NPCs prematurely exit the cell cycle, as evidenced by an increased frequency of cells in G1/G0 phase (Hanashima et al., 2002, Hanashima et al., 2004; Xuan et al., 1995). In humans, overexpression of FOXG1 is observed in glioblastoma (Chen et al., 2018; Seoane et al., 2004) and promotes brain tumor growth (Verginelli et al., 2013). These data provide clinically relevant evidence that FOXG1 dosage has a severe impact on NPC proliferation (Hettige and Ernst, 2019).Recent advances in stem cell biology have allowed for in vitro human modeling of cortical brain development, preserving major developmental milestones (Ardhanareeswaran et al., 2017). Stem cells derived from a somatic cell (Takahashi et al., 2007) are differentiated to ectoderm and then neuralized using factors known to be present at critical time points in neurodevelopment (Bell et al., 2019). The temporal sequence of NPC proliferation and differentiation is determined by the sequential activation of growth factors and other small molecules (Kohwi and Doe, 2013) provided by the experimenter. Thus, important mechanisms such as those involved in the switch from proliferation to differentiation of NPCs can be studied in cells that meet defining characteristics of a cell type, such as expression of markers or physiological properties of the cell.To date, a handful of studies have used induced pluripotent stem cells (iPSCs) to model FOXG1 syndrome. In 2016, Patriarchi et al. generated iPSC-derived neurons from two FOXG1+/− cases and observed an imbalance in excitatory/inhibitory synaptic protein expression (Patriarchi et al., 2016). However, these data did not explore the dynamics of FOXG1 dose as NPCs develop. To investigate FOXG1 dose, Zhu et al., 2019 used a tunable degron motif to modify the expression of endogenous FOXG1, and they observed an increased G1 phase of NPCs and increased proportion of GABAergic interneurons after NPC differentiation (Zhu et al., 2019). What is currently missing is a comprehensive, robust investigation of the role of FOXG1 mutations that cause microcephaly. Here, we use human patient cells and engineered lines to assess how changes in FOXG1 dose might lead to cellular phenotypes relevant to disease.
Results
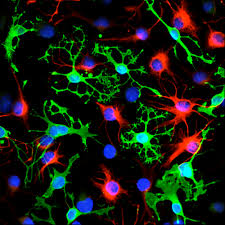
Identifying the timing of FOXG1 expression in developing neurons
In rodent neurodevelopment, Foxg1 is expressed at E8.5 (Tao and Lai, 1992), but the point at which FOXG1 is turned on for human in vitro differentiation protocols remains unknown. To examine the dynamics of FOXG1 expression in human neurodevelopment, we reprogrammed somatic cells into pure iPSC colonies while simultaneously inserting a tdTomato tag onto endogenous FOXG1 (Bell et al., 2019). iPSCs were induced to become NPCs over a 12-day differentiation period after which cells were suspended and purified for 2–3 days for NPC purification (Figure 1A ). To ensure the successful differentiation of NPCs, iPSC lines underwent extensive quality-control screening, including copy-number variation (CNV) analysis and endogenous-marker staining (Figure 1B). We define NPCs as intermediate forebrain progenitors that express specific neural stem cell markers (Figure 1C) and proliferate in maintenance medium with the potential to differentiate into forebrain neurons. Forebrain cortical neurons are defined by their morphology and expression of the neuronal markers MAP2 and TUJ1 (Figure 1D). Using a purified iPSC line that successfully integrated the tdTomato tag to endogenous FOXG1 (Figure 1E), we live imaged cells over the 12-day NPC induction period and for 6 days post-differentiation in NPC maintenance medium without mechanical purification. This allowed us to assess direct effects of culture conditions and compare across time points (i.e., without passaging cells). Beginning at day 5, we observed the presence of a red signal in some differentiating iPSC colonies. A higher proportion of cells became noticeably red at day 7 (Figure 1F), coinciding with the fact that we could detect neural stem cell markers, NESTIN and SOX1, at this same time point (Figure 1G). As the colonies continued to differentiate into NPCs, we observed that the majority of the cells displayed a red signal by approximately day 12. After 12 days in neural induction medium, cells were switched to NPC maintenance medium. Notably, the longer NPCs were kept in maintenance medium, the fainter the red signal became (Figure 1F). This live-cell reporter assay suggests that FOXG1 is induced relatively early in the stem cell neural-induction process and that it is likely at peak expression here due to exposure to factors in the induction medium. FOXG1 levels are substantial but decrease the longer cells are maintained and passaged in maintenance medium. To confirm our findings from the cell reporter assay, we validated results using three independent and healthy stem cell lines to confirm FOXG1 changes during neural induction. After first ensuring that the antibody could detect FOXG1 (Figure S1), we detected FOXG1 as early as day 7 during neural induction. While there is cell-line variability, FOXG1 is nevertheless expressed at relatively early stages during neural induction.