Abstract
Transcriptional regulator MtrR inhibits the expression of the multidrug efflux pump operon mtrCDE in the pathogenic bacterium Neisseria gonorrhoeae. Here, we show that MtrR binds the hormonal steroids progesterone, β-estradiol, and testosterone, which are present at urogenital infection sites, as well as ethinyl estrogen, a component of some hormonal contraceptives. Steroid binding leads to the decreased affinity of MtrR for cognate DNA, increased mtrCDE expression, and enhanced antimicrobial resistance. Furthermore, we solve crystal structures of MtrR bound to each steroid, thus revealing their binding mechanisms and the conformational changes that induce MtrR.
Introduction
The pathogen Neisseria gonorrhoeae is a Gram-negative diplococcus that colonizes genital, rectal, and oral mucosa1. As a strict human pathogen, N. gonorrhoeae is finely adapted for survival in its sole natural host and infects over 85 million individuals (both sexes) each year worldwide2 and infections in the US are far more prevalent in women than men3. No vaccine is available, and reinfection by identical gonococcal strains is possible1,4. After successful colonization by gonococci (GC), a female patient may experience complications arising from infection including pelvic inflammatory disease, ectopic pregnancy, and infertility1. Neonatal blindness caused by maternal transmission during childbirth can also occur without appropriate preventative measures1.
Since the late 1930’s with the availability of sulfonamides followed by penicillin, antibiotics have been used to treat gonococcal infections, but their effectiveness has decreased with the rise of multidrug resistance (MDR)5. To date, N. gonorrhoeae has developed resistance to sulfonamides, macrolides, aminoglycosides, beta-lactams, tetracyclines and fluoroquinolones1,6. Currently, the sole empiric treatment for gonococcal infections in the US is ceftriaxone but alarmingly, ceftriaxone-resistant strains have been identified in Denmark, France, Japan, Thailand, and the United Kingdom7,8,9,10,11,12. This consistent rise in MDR indicates the possibility of untreatable strains of N. gonorrhoeae and has prompted the CDC (Centers for Disease Control and Prevention) to flag this bacterium as an urgent public health threat. Thus, a detailed molecular understanding of gonococcal MDR mechanisms and their regulation are crucial to inform public health decisions7.
N. gonorrhoeae, like most pathogens, contains a number of mechanisms that lead to antibiotic resistance including β-lactam antibiotic resistance by antibiotic modifying proteins13, ribosome protective proteins14, mutations in penicillin binding proteins6, and reduced permeability of cell walls by outer membrane proteins6,15. Significantly, the direct export of antibiotics and toxic molecules by multidrug efflux pumps is a major contributor to gonococcal survival and MDR. Multidrug efflux pumps decrease the cytosolic and periplasmic concentration of antibiotics and cytotoxins by pumping them into the extracellular milieu16. These systems can recognize and efflux a wide variety of chemically and structurally dissimilar molecules16. The best characterized multidrug efflux system of N. gonorrhoeae is the MtrCDE tripartite system, a member of the Resistance-Nodulation-Division (RND) family1. Studies investigating MDR in N. gonorrhoeae report significant increases in gonococcal susceptibility to antibiotics when the MtrCDE system is impaired or deleted17. Overexpression of the MtrCDE efflux system due to cis- or trans-acting regulatory systems is a leading cause of gonococcal resistance to hydrophobic agents (HAs), fatty acids, and even human antimicrobial peptides such as LL-3717,18,19. However, the energetic cost of synthesizing efflux pumps is significant, and their expression is regulated tightly leading to optimal cell viability20.
The mtrCDE operon is regulated by the multiple transferable resistance repressor, MtrR, which also acts as a global transcriptional regulator in N. gonorrhoeae17. Beyond its regulation of the expression of the mtrCDE operon, MtrR directly represses rpoH, which encodes a secondary sigma factor that controls the stress response in gonococci21,22,23. MtrR can regulate these genes by binding a degenerate consensus sequence upstream of their coding regions thus blocking access of RNA polymerase to the promotor17,21. Upon binding to cytotoxic molecules and likely sensing oxidative stress, MtrR undergoes a conformational change to relieve repression of its target genes (Fig. 1A)18.
A member of the ubiquitous TetR family, MtrR is entirely alpha helical and comprises a helix-turn-helix (HTH) DNA binding motif and a C-terminal ligand binding/dimerization domain (Fig. 1B, C)18. Mutations in the mtrR gene or its cognate promoters are present in many clinical isolates resistant to antimicrobials and antibiotics24. Recent structural and biochemical studies on the MtrR-rpoH and MtrR-mtrCDE operator complexes reveal the basis of loss of function mutations in the HTH DNA binding motif that abolish regulation of these genes by MtrR25.
Despite these initial studies, little is known about the induction and ligand recognition mechanisms of MtrR by innate human inducers. Previous work from our laboratories has shown that MtrR binds and is induced by the bile salts chenodeoxycholate (CDCA) and taurodeoxycholate (TDCA), found at extra-urogenital GC infection sites, but not glycocholate18. However, physiologically relevant inducers of MtrR that are found at urogenital sites, which form the vast majority of the sites of gonococcal infection, are not known, posing a significant gap in our understanding of how MtrR contributes to MDR and gonococcal survival in the reproductive tract. To address this critical lack of knowledge, we carried out a series of structural, biochemical, and cellular experiments to elucidate the binding and induction mechanisms of MtrR by female and male steroidal hormones as they are physiologically relevant molecules that would confront gonococci during infection. In the female reproductive tract, gonadol hormones are responsible for the coordination of immune cell function, vaginal microbiome, and epithelial cell architecture during menstration to optimize maternal protection against pathogen infection and also fetal implantation and survival26,27,28. This results in a “window of vulnerability” where the immune protection is dampened in the reproductive tract in order to optimize sperm and fetal survival, which can be taken advantage of by pathogens26,27. Thus, pathogens can utilize hormones to regulate gene expression involved in cytotoxic stress defenses allowing host infection26,29,30,31,32,33,34. Mechanisms for sensing and coping with cytotoxic stresses are crucial for survival of all organisms, particularly obligate human pathogens like N. gonorrhoeae that are routinely exposed to cytotoxic attacks by the host and inhabit an environment that is well equipped with antimicrobial defense mechanisms. Elucidation of the functions of MtrR will contribute to the understanding of MDR in N. gonorrhoeae, and transcriptional regulation that promotes pathogen survival. Here, we show that binding of human steroidal hormones results in a comformational change in the MtrR structure rendering it incompatible with DNA binding, thus revealing the structural mechanism of MtrR induction.
Results
Steroidal hormones bind MtrR and reduce its DNA-binding affinity
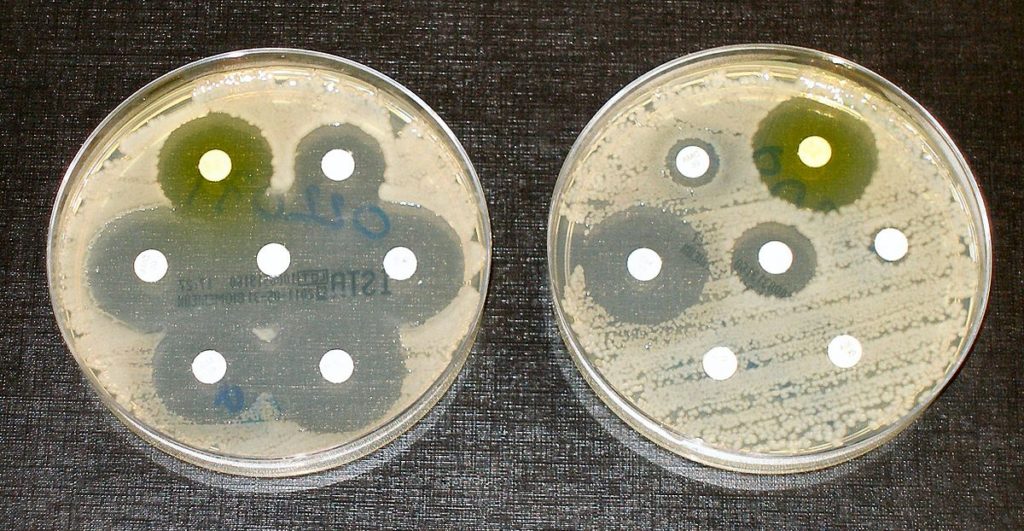
We first investigated the ability of MtrR to bind selected ligands. Because bacterial regulatory proteins of efflux systems are commonly induced by the substrates of the corresponding pump, allowing increased expression and resistance under cytotoxic stresses, we hypothesized that some ligands of MtrCDE are MtrR inducers35,36,37. Previous work has shown that mutations of the mtrD gene result in a significant decrease in the minimal inhibitory concentration (MIC) of progesterone (PTR) in N. gonorrhoeae38. Other physiologically relevant candidates of MtrR inducers include the gonadal steroids β-estradiol (EST) and testosterone (TES), which, in addition to PTR, have been shown to arrest N. gonorrhoeae growth39. Of these three, PTR is the most effective in growth inhibition, highlighting this compound as a potent protective agent against gonococcal infection. The effect of PTR may also explain the higher occurrence of asymptomatic gonorrheal infection in women than men39. Mutations in the mtrR gene also confer increased susceptibility to PTR in plated cells as well as decreased survival in the lower genital tract of female mice40. Additionally, female mice models are most susceptible to gonococcal infection during periods of the menses cycle when EST levels are highest41, and mice treated with EST showed significantly increased susceptibility to disseminated gonococcal infection42.
We conducted isothermal titration calorimetry (ITC) studies on EST, PTR, and TES to determine their affinities for MtrR. These studies revealed that MtrR bound these steroids with equilibrium dissociation constants (Kd) of 2.75 ± 0.7 µM (PTR), 1.67 ± 1.1 µM (EST), and 2.26 ± 0.3 µM (TES) (Fig. 2A), whilst the chemical precursor cholesterol, the stress hormone cortisol, and the antibiotic azithromycin (AMR), show no binding (Fig S1). The stoichiometry for EST and TES is one molecule of steroid per MtrR monomer and intriguingly one molecule of PTR per MtrR dimer. In addition to these three gonadal steroids, we also tested the binding of ethinyl estradiol (NDR), which is a synthetic estrogen used in some hormonal contraceptives43, and found that it binds MtrR with a Kd of 0.94 ± 0.6 µM (Fig. 2A). These results show the substantial affinity and specificity of MtrR for steroidal hormones. To determine if these identified host ligands result in the biochemical induction of MtrR, we measured the binding affinity of MtrR for the rpoH and mtrCDE operators in the presence and absence of 125 µM PTR, EST, TES, or NDR (in 1% methanol (MeOH)) using a DNA binding fluorescence polarization-based assay (FP). Results from these FP experiments showed up to a 13-fold and 3-fold decrease in binding affinity of MtrR for the rpoH and mtrCDE operator, respectively, in the presence of each steroid compared to binding of MtrR to these sites in the presence of 1% MeOH alone (Fig. 2B, S2). At higher concentrations, MtrR begins to bind nonspecifically to the DNA, possibly explaining why MtrR shows a more modest fold change in binding affinity for the mtrCDE operator compared to rpoH. These data support the hypothesis that gonadal steroids are physiologically relevant inducers of MtrR regulated genes….