Abstract
The development of new immunotherapies to treat the inflammatory mechanisms that sustain atherosclerotic cardiovascular disease (ASCVD) is urgently needed. Herein, we present a path to drug repurposing to identify immunotherapies for ASCVD. The integration of time-of-flight mass cytometry and RNA sequencing identified unique inflammatory signatures in peripheral blood mononuclear cells stimulated with ASCVD plasma. By comparing these inflammatory signatures to large-scale gene expression data from the LINCS L1000 dataset, we identified drugs that could reverse this inflammatory response. Ex vivo screens, using human samples, showed that saracatinib—a phase 2a-ready SRC and ABL inhibitor—reversed the inflammatory responses induced by ASCVD plasma. In Apoe−/− mice, saracatinib reduced atherosclerosis progression by reprogramming reparative macrophages. In a rabbit model of advanced atherosclerosis, saracatinib reduced plaque inflammation measured by [18F]fluorodeoxyglucose positron emission tomography–magnetic resonance imaging. Here we show a systems immunology-driven drug repurposing with a preclinical validation strategy to aid the development of cardiovascular immunotherapies.
Main
ASCVD is the leading cause of death worldwide1,2, but the development of new cardiovascular drugs has lagged compared with the advancements made for other complex diseases conditions, such as cancer3. The current standard of care for ASCVD is to lower lipid levels and control other cardiovascular risk factors (such as diabetes, hypertension)4, but these approaches do not directly address the underlying inflammatory mechanisms of the disease5. Indeed, since the discovery of lipid-lowering statins6 and the recent PCSK9 inhibitors7, drug innovation in the field has been stagnant. This is in part due to the failures of traditional drug discovery efforts8 and the substantial investments required for large, outcome-driven phase 3 clinical trials with long-term follow-up for outcomes in individuals with ASCVD9. Immunomodulatory treatments are a promising approach to reduce the residual risk of stroke and myocardial infarction in individuals with ASCVD.
Drug repurposing is a cost-effective approach to rapidly transition existing drugs into the clinic for new indications3. Immunomodulatory drug repurposing studies have proven successful in recent years. For example, the knowledge that interleukin 1β (IL-1β) drives inflammation in ASCVD led to the successful design of the Canakinumab Anti-inflammatory Thrombosis Outcome Study10. In 2017, this seminal study proved that targeting inflammation reduces the risk for secondary cardiovascular events in patients, but US Food and Drug Administration approval for the use of IL-1β humanized neutralizing antibody canakinumab was not granted because the data were considered insufficient to justify routine use in patients with ASCVD. In 2019, the Colchicine Cardiovascular Outcomes Trial11 showed that the anti-inflammatory drug colchicine achieved similar efficacy on cardiovascular outcomes for patients with ASCVD.
However, other drug repurposing-based clinical trials were either unsuccessful or showed that a one-size-fits-all immunotherapeutic approach is unattainable due to variability in patient responses. For example, the Cardiovascular Inflammation Reduction Trial12 showed no efficacy of low-dose methotrexate, the gold-standard therapy for rheumatic arthritis, in patients with ASCVD. Moreover, patient outcomes on colchicine treatment proved more mixed than initially recognized, whereas low-dose colchicine reduced composite cardiovascular endpoints in patients with stable coronary artery disease (CAD) in two trials13,14. In the Colchicine in Patients with Acute Coronary Syndromes trial, patients had higher mortality and experienced no reduction in cardiovascular outcomes at 12 months15. These studies suggest that further investigation of immunomodulatory therapies in ASCVD is warranted.
Multifactorial disorders like ASCVD are modulated by complex gene and protein regulatory networks that span the interactions between different cell types16,17. The advent of single-cell analyses and systems biology have revealed heterogeneous immune alterations in the blood and in atherosclerotic vascular tissues of patients, and uncovered immune cell transcriptional alterations in plaques18,19. Harnessing system-level analyses offers the promise of discovering drugs that may restore dysregulated immune responses in ASCVD. Here we present a path to a drug repurposing approach that combines innovative systems immunology-driven drug repurposing with a functional screen that is applied directly to human samples. In conjunction with a rigorous preclinical validation platform in animal models, this system can aid the clinical translation of existing drugs with new cardiovascular indications tailored to individual patients.
Results
Phospho-CyTOF identifies immune alterations in patients with atherosclerosis
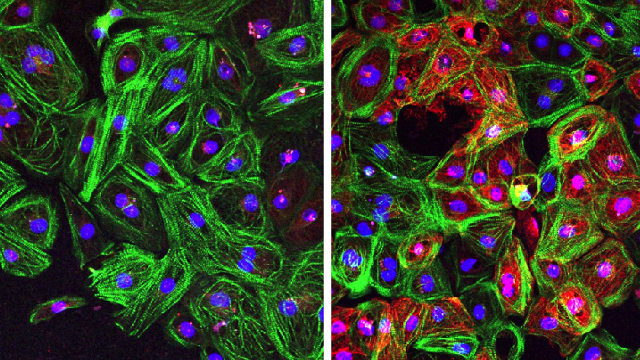
To characterize functional dysregulation of immune cells in human atherosclerosis, we isolated peripheral blood mononuclear cells (PBMCs) from patients with carotid atherosclerosis (Supplementary Table 1) and exposed them to either autologous plasma (referred to as atheroplasma or atherosclerotic plasma) or plasma from healthy donors (referred to as healthy plasma). Using phospho-cytometry by time-of-flight (phospho-CyTOF), a mass cytometry method to study intracellular phospho signaling pathways at the single-cell level, we then interrogated the activation of major immune cell signaling pathways across all main immune populations (Fig. 1a). Using viSNE, a visualization tool for high-dimensional single cell data, we visualized ten major immune cell populations (B cells, basophils, CD1c+ dendritic cells (DCs), CD4+ T cells, CD8+ T cells, CD14+ and CD16+ monocytes, natural killer cells, natural killer T cells and plasmacytoid DCs) on the basis of canonical marker expression patterns (Extended Data Fig. 1a,b). Next, to identify intracellular signaling pathways activated within each population, we quantified the phosphorylation of ten intracellular proteins (IκBɑ (nuclear factor of κ light chain polypeptide gene enhancer in B cells inhibitor-ɑ), CREB (cAMP-response element binding protein), ERK1/2 (extracellular signal-regulated kinase 1 and 2), MAPKAPK2 (mitogen-activated protein (MAP) kinase-activated protein kinase 2), p38 (p38 MAP kinase), PLCG2 (phospholipase Cγ2), S6 (ribosomal protein S6), STAT1 (signal transducer and activator of transcription 1), STAT3 and STAT5) across this immunological map. Data were integrated to derive 100 cell type–phosphoprotein pairs that were compared across each condition, revealing the greatest immune activation in CD14+ monocytes and CD1c+ DCs (Fig. 1b). Specifically, compared with healthy plasma, exposure to autologous atherosclerotic plasma induced the phosphorylation of CREB, p38, ERK1/2, MAPKAPK2 and S6 in CD14+ monocytes and CD1c+ DCs (Fig. 1c–e, Extended Data Fig. 1c–e). Other immune cell types responded to autologous atherosclerotic plasma, including CD4+ and CD8+ T cells, but both the number of activated phosphosites and the magnitude of their activation was lower than in CD14+ monocytes and CD1c+ DCs (Fig. 1b). These results suggest that, in patients with atherosclerotic disease, plasma from these patients induces a strong and specific innate immune cell signaling responses in circulating inflammatory cells…