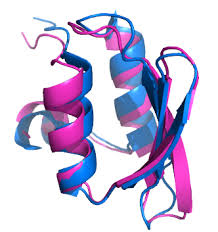
Structural basis of client specificity in mitochondrial membrane-protein chaperones
Abstract Chaperones are essential for assisting protein folding and for transferring poorly soluble proteins to their functional locations within cells. Hydrophobic interactions drive promiscuous chaperone-client

