Abstract
Chaperones are essential for assisting protein folding and for transferring poorly soluble proteins to their functional locations within cells. Hydrophobic interactions drive promiscuous chaperone-client binding, but our understanding of how additional interactions enable client specificity is sparse. Here, we decipher what determines binding of two chaperones (TIM8·13 and TIM9·10) to different integral membrane proteins, the all-transmembrane mitochondrial carrier Ggc1 and Tim23, which has an additional disordered hydrophilic domain. Combining NMR, SAXS, and molecular dynamics simulations, we determine the structures of Tim23/TIM8·13 and Tim23/TIM9·10 complexes. TIM8·13 uses transient salt bridges to interact with the hydrophilic part of its client, but its interactions to the transmembrane part are weaker than in TIM9·10. Consequently, TIM9·10 outcompetes TIM8·13 in binding hydrophobic clients, while TIM8·13 is tuned to few clients with both hydrophilic and hydrophobic parts. Our study exemplifies how chaperones fine-tune the balance of promiscuity versus specificity.
INTRODUCTION
Cellular survival and function fundamentally rely on an intact proteome. Proteins within cells need to be correctly folded to their functional conformation and be present at the cellular location where they function. Chaperones play a central role in maintaining this cellular protein homeostasis (1), either by helping other proteins to reach their functional three-dimensional (3D) structure after synthesis, by transporting them across the cytosol or organelles, or by sustaining their native fold along their lifetime. More than 20,000 different proteins are required to fulfill the functions of human cells, and it is believed that the majority rely on chaperones to reach and maintain their native fold (2). Given the diversity of the client proteins, many chaperones promiscuously interact with tens of different “client” proteins that may differ widely in size, structure, and physicochemical properties. However, the need for efficient binding and refolding of their clients also calls for some degree of specificity. Chaperones operate at this delicate balance of promiscuity and specificity to their clients. The interactions that determine the chaperone-client specificity are only partly understood.
Hydrophobic interactions play a crucial role for chaperone interactions, as most chaperones bind to hydrophobic patches on their clients and shield them from aggregation. Electrostatic charges also play a role in some chaperone complexes (3). The interaction motifs recognized by different chaperones differ by their physicochemical properties (4). For example, for interacting with the Hsp70 chaperone family, Ile, Phe, Leu, and Val residues are particularly important (5, 6); the SecB chaperone recognizes nine-residue-long stretches enriched in aromatic and basic residues (7); the chaperone Spy uses longer-range charge interactions for the formation of an initial encounter complex, followed by more tight binding mediated by hydrophobic interactions (8), whereby structurally frustrated sites on the client protein are particularly prone to binding (9).
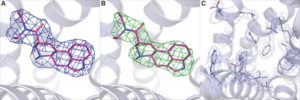
Our understanding of the underlying principles of chaperone-client interactions is hampered by the lack of atomic-level views onto the structure and dynamics of these complexes. Their inherently dynamic and often transient nature represents a substantial experimental challenge toward structural characterization. Only a very limited number of chaperone complex structures have been reported [reviewed in (10)]. The modes of interactions that they revealed range from rather well-defined binding poses of client polypeptides in the chaperone’s binding pockets, reminiscent of complexes formed by globular proteins, to highly flexible ensembles of at least partly disordered conformations (“fuzzy complexes”). In the latter, a multitude of local chaperone-client interactions may result in a high overall affinity despite the low affinity and short lifetime of each individual intermolecular contact.
Multiple molecular chaperones are present in the cell with mutually overlapping functions and “clientomes” (2, 11, 12). It is poorly understood, however, whether a given client protein adopts a different conformation (or ensemble of conformations) when it is bound to different chaperones, and if different clients, when bound to a given chaperone, all show similar conformational properties. α-Synuclein appears to have similar interaction patterns with six different chaperones (13); outer membrane proteins (OmpA, OmpX, and FhuA) have similar properties—essentially fully unfolded—when bound to SurA and Skp chaperones (14, 15), at least as judged by their nuclear magnetic resonance (NMR) fingerprint spectra. Phosphatase A displays an extended dynamic conformation, but well-defined binding poses of its interacting parts, when bound to trigger factor (16), Hsp40 (17), or SecB (18). Thus, while these reports suggest that a given protein adopts similar properties on different chaperones, the scarcity of data and the absence of a direct comparison of complex structures leave open which interactions may confer specificity.
A pair of “holdase” chaperone complexes of the mitochondrial intermembrane space (IMS), TIM8·13 and TIM9·10, are structurally highly similar but have different substrate binding preferences. These chaperones transport precursors of membrane proteins with internal targeting sequence (henceforth denoted as “precursors”) to the membrane-insertase machineries in the inner membrane (TIM22) and outer mitochondrial membranes (SAM) (19). The TIM chaperones form hetero-hexameric structures of ca. 70 kDa, composed of an alternating arrangement of Tim9 and Tim10 or Tim8 and Tim13. TIM9·10 is essential to cellular viability (20–22); even single-point mutations in Tim9 or Tim10 that keep the chaperone structure intact but affect precursor protein binding can impair yeast growth and cause lethality (23). Although TIM8·13 is not essential in yeast (24), yeast cells depleted of Tim8 and Tim13 show conditional lethality (25). In addition, mutations in the human Tim8a protein have been identified as the cause of a neurodegenerative disorder known as Mohr-Tranebjærg syndrome or deafness-dystonia-optic neuropathy syndrome (26, 27).
In vivo experiments, predominantly in yeast, have identified mitochondrial membrane proteins whose biogenesis depends on small TIM chaperones. TIM9·10 is believed to interact with all members of the mitochondrial carrier (SLC25) family, which comprises more than 50 members in humans, such as the adenosine diphosphate (ADP)/adenosine triphosphate (ATP) carrier (Aac in yeast); furthermore, TIM9·10 transports the central components of the TIM22 and TIM23 insertion machineries (Tim23, Tim17, and Tim22) as well as outer membrane β barrel proteins (28). TIM8·13 has a narrower clientome and was shown to bind the precursors of the inner membrane proteins Tim23 (25, 29, 30) and Ca2+-binding aspartate-glutamate carriers (31), as well as the outer membrane β barrel proteins VDAC/Porin, Tom40 (32), and Tob55/Sam50 (33). There is evidence that TIM8·13 does bind neither the inner membrane protein ADP/ATP carrier (Aac) nor Tim17 (25). The inner membrane proteins that have been reported to interact with TIM8·13 have a hydrophilic domain in addition to transmembrane (TM) domains (fig. S1), but this does not hold true for the outer membrane β barrels. Thus, the mechanisms by which TIM8·13 binds its clients remain unclear.
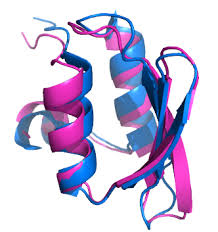
Recently, we obtained the first structure of a complex of a small TIM chaperone, TIM9·10, with the mitochondrial guanosine diphosphate (GDP)/guanosine triphosphate (GTP) carrier (Ggc1) (23). The structure, composed of two chaperone complexes holding one precursor protein, revealed a highly dynamic ensemble of Ggc1 conformers that form multiple short-lived and rapidly interconverting (<1 ms) interactions with a hydrophobic binding cleft of the chaperone (fig. S2). The TIM9·10-Ggc1 complex can be described as a “fuzzy complex,” in which the high overall affinity is driven by a multitude of individually weak interactions with the hydrophobic TM parts of its clients.
To understand what confers specificity in the mitochondrial IMS chaperone system, we studied chaperone complexes of TIM9·10 and TIM8·13 with two precursor proteins, the Ggc1 and the insertase component Tim23. In their native state, Ggc1 comprises six TM helices without soluble domains, and Tim23 comprises four TM helices and a ca. 100-residue-long soluble IMS domain (Fig. 1A). By solving the complex structures of the two chaperone complexes holding Tim23, we reveal that the differential specificity of the two chaperones is based on an interplay of hydrophobic and hydrophilic interactions, which leads to different conformational properties of the precursor protein bound to these chaperones.