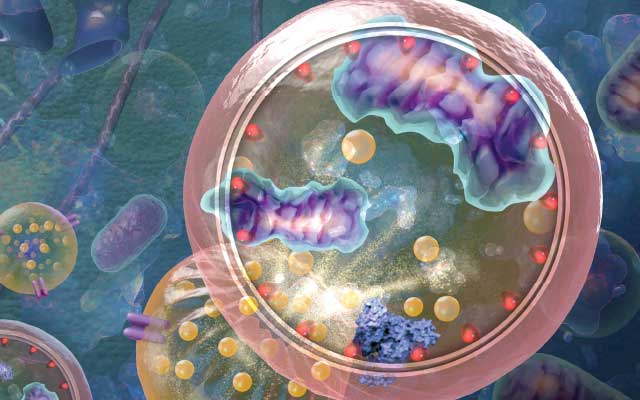
In the mid-1950s, Sam Clark Jr. of the School of Medicine at Washington University in St. Louis looked through his electron microscope at newborn-mouse kidneys and spotted something he’d never seen before. As he later described it, there appeared to be membrane-bound structures within the cytoplasm of the kidney cells. Intriguingly, these structures seemed to contain altered mitochondria.1
Soon after Clark published his observations, several independent researchers supported his findings. These included the Albert Einstein College of Medicine’s Alex Novikoff, who used the term cytolysome for the structures. “Within these cytolysomes remarkable events are in progress . . .” he and his colleague Edward Essner wrote in 1962.2 “Cytoplasm has somehow found its way inside the droplets and is apparently in the process of digestion.”
These were the first descriptions of what is known today as macro-autophagy (hereafter referred to as autophagy). The term is derived from the Greek “auto,” meaning self, and “phagein,” meaning to eat. During autophagy, the cell consumes parts of itself in a regulated manner.
The hallmark of autophagy is the formation of a transient double-membrane structure termed a phagophore. In contrast to secretory transport vesicles, which bud off from an organelle with cargo already enclosed within, the phagophore acquires cargo during its assembly. It may form de novo in the cytoplasm as a free-standing structure, or it may be in contact with an organelle such as the endoplasmic reticulum. The phagophore expands sequentially, providing tremendous flexibility with regard to cargo capacity. As it expands, it sequesters cytoplasmic components, including proteins, lipids, and even entire organelles. Once its payload is secured, the phagophore closes and matures into an autophagosome, with the sequestered cargo now enclosed in the lumen of this compartment. The autophagosome then delivers the cargo, via membrane fusion, to the lytic compartments—vacuoles in fungi and plants and lysosomes in metazoans—for degradation and recycling. It was the autophagosome that captured the attention of Clark and Novikoff more than half a century ago.
TYPES OF AUTOPHAGY
Autophagy can be divided into two broad categories, selective and nonselective, based on the nature of what’s being eaten. The most extensively studied and characterized form of autophagy is macroautophagy, which involves the delivery of cellular components to the lysosome (or vacuole in fungi and plants) via a double membrane–bound structure. Two other forms, beyond the scope of this article, are microautophagy and chaperone-mediated autophagy. During microautophagy, the lysosomal membrane invaginates and sequesters nearby cellular materials for degradation and recycling. Chaperone-mediated autophagy, in contrast, is a specialized protein-degradation process involving dedicated lysosomal transporters.
Today, autophagy is recognized as a critical process for maintaining cellular homeostasis, as well as for responding to stressors, such as nutrient deficiency, which may potentially compromise cell survival. When a cell is exposed to such stressors, autophagy, which occurs constitutively at low levels to balance the constant synthesis of biomolecules, is strongly upregulated. This upregulation increases sequestration and degradation of portions of the cell, releasing macromolecules back into the cytosol to power essential metabolic reactions and generate energy.
The contribution of autophagy to cellular health under both normal and stress conditions implies important physiological and pathological roles for this tightly regulated and precisely orchestrated process. Indeed, autophagy has been found to be instrumental during the course of mammalian development. Additionally, recent research has discovered that autophagy is a critical modulator of a wide range of diseases and disorders. Probing the involvement of autophagy in development and disease is crucial for a more complete understanding of the pathway’s roles, and could have implications for maintaining health or treating disease. While we partially understand its overall morphology and function, information about several steps in this intricate pathway are still emerging.
Mechanisms of autophagy
Several catabolic pathways in the cell break down large molecules. Notably, the conjugation of a small protein called ubiquitin to another cellular protein—often followed by the sequential addition of ubiquitin molecules to generate a polyubiquitin chain—can tag that protein for degradation by the proteasome, resulting in the release of amino acids. (See “The Proteasome: A Powerful Target for Manipulating Protein Levels,” The Scientist, May 2017.) Similar degradation mechanisms exist for other biological polymers such as carbohydrates and lipids.
So what makes autophagy unique? The answer lies in the flexibility of autophagosome size and cargo selection. Autophagy can promote degradation en masse for a large number and variety of substrates, enabling cells to quickly and efficiently generate recycled basic building materials in the face of a wide range of nutritional deficiencies. Additionally, autophagy is the only pathway that is capable of degrading entire organelles, either randomly or in a targeted fashion—a critical process for maintaining homeostasis in the complex landscape of the eukaryotic cell.
Autophagy is tightly regulated to ensure that it is ramped up only when required, and then in a timely manner. The central metabolic sensor of the cell, the TOR complex 1 (TORC1, or MTORC1 in mammals), is sensitive to the availability of amino acids and growth factors, and inhibits autophagy induction when these components are abundant. When cells are starved of these molecules, TORC1/MTORC1 is inactivated, promoting an increase in autophagy. Meanwhile, other molecular regulators monitor cells for the status of various nutrients, such as glucose, or for energy in the form of ATP, and trigger autophagy when such nutrients or metabolites reach critically low levels.
Once autophagy is initiated, several autophagy-related (Atg) proteins act together to coordinate the formation of the phagophore and the subsequent steps of autophagy. Yeast ATG genes were discovered in the 1990s, transforming autophagy research, which had been largely descriptive, to being strongly mechanistic at the molecular level. Experiments using the genetically tractable budding yeast Saccharomyces cerevisiae played a major role in helping scientists decipher the basic mechanism of autophagy. Research in other organisms followed shortly afterward, revealing a remarkable evolutionary conservation in the nature and function of the autophagy machinery from yeast to human. (See illustration here.)
While the overall process of auto-phagy is now somewhat clear to scientists, the field is still hard at work trying to find and fit in many missing pieces of the puzzle. The donor of the membrane for the autophagosome, for example, has not been concretely established. (See “The Enigmatic Membrane,” The Scientist, February 2012.) Similarly, we do not fully understand how phagophore expansion is regulated, or what dictates the frequency of autophagosome generation. Even more questions arise when we consider that many types of autophagy are highly selective, and the initiation and regulation of these processes remain mysterious. Further understanding of these selective pathways is crucial because they are intimately linked to embryonic development, healthy growth, and human disease.
Autophagy in development
In the late 1970s, Richard Lockshin of St. John’s University in New York and colleagues demonstrated that auto-
phagy occurs during insect metamorphosis.3 A decade and a half later, researchers showed that mutant yeast cells defective in autophagy do not sporulate.4 These were the first hints that autophagy may play a role in organismal development, but only relatively recently have researchers begun to elucidate the process’s contributions to cellular differentiation and development in metazoans….