Abstract
Once considered as a “metabolic waste,” lactate is now recognized as a major fuel for tricarboxylic acid (TCA) cycle. Our metabolic flux analysis reveals that skeletal muscle mainly uses lactate to fuel TCA cycle. Lactate is transported through the cell membrane via monocarboxylate transporters (MCTs) in which MCT1 is highly expressed in the muscle. We analyzed how MCT1 affects muscle functions using mice with specific deletion of MCT1 in skeletal muscle. MCT1 deletion enhances running performance, increases oxidative fibers while decreasing glycolytic fibers, and enhances flux of glucose to TCA cycle. MCT1 deficiency increases the expression of mitochondrial proteins, augments cell respiration rate, and elevates mitochondrial activity in the muscle. Mechanistically, the protein level of PGC-1α, a master regulator of mitochondrial biogenesis, is elevated upon loss of MCT1 via increases in cellular NAD+ level and SIRT1 activity. Collectively, these results demonstrate that MCT1-mediated lactate shuttle plays a key role in regulating muscle functions by modulating mitochondrial biogenesis and TCA flux.
INTRODUCTION
As the end product of glycolysis, lactate has been the center of controversy in biology and exercise physiology (1). Lactate was originally discovered by Swedish apothecary and chemist Carl Wilhelm Scheele in 1780 in sour milk. Within the physiological pH range in the body, lactate is more than 99% dissociated into lactate anions (La−) and protons (H+). For over 200 years and even up to now, lactate was arguably considered “metabolic waste” in “the muscles of hunted age” causing fatigue and muscle soreness. This paradigm was mainly formed in early days by experiments showing “hypoxia/anoxia (low O2) evokes high lactate” and vice versa “high lactate denotes low O2” (1). The discoveries that skeletal muscle and many other tissues/cells can produce lactate under fully aerobic conditions led George Brooks to propose a “lactate shuttle” theory in the mid-1980s (2, 3). The core of the lactate shuttle theory holds that lactate is an energy intermediate that is formed in cells/tissues with high glycolytic activity and then consumed by cells/tissues with high oxidative activity (3, 4). Lactate is now considered an important energy source for oxidative phosphorylation in cells/tissues, such as oxidative muscle fibers, as well as a precursor for gluconeogenesis (in the liver) and glycogenesis (in liver and skeletal muscle) (1, 4). The importance of lactate as an energy source was highlighted by a recent discovery with flux analysis illustrating that lactate is a preferred fuel feeding the TCA cycle to glucose (5, 6). This finding supports earlier proposals that intracellular lactate shuttle is involved in the utilization of lactate as a fuel for TCA cycle via mitochondria lactate oxidation complex (mLOC) (3, 7, 8). In addition to serving as a fuel source, lactate can initiate signaling cascade in certain cells upon binding to membrane receptors, exemplified by the discovery that lactate inhibits lipolysis of adipocytes via membrane hydroxycarboxylic acid receptor 1 receptor (9). Lately, lactate was found to execute epigenetic regulation on gene expression through lactylation of histone and thereby regulate macrophage functions (10).
Cell-cell and intracellular communication of lactate can be accomplished by membrane transporters. A series of work mainly in the Halestrap laboratory discovered that the transmembrane movement of lactate is carried out by members of the solute carrier family 16 (SLC16) (11). Within this family, monocarboxylate transporters (MCTs) 1 to 4 (encoded by Slc16a1, Slc16a7, Slc16a8, and Slc16a3, respectively) are responsible for transport of lactate, pyruvate, and ketone bodies. MCT-mediated monocarboxylate transport is concentration dependent, adenosine triphosphate (ATP) independent, saturable, stereospecific, competitively inhibited by other monocarboxylates, sensitive to temperature, and stimulated by [H+] gradients (1). MCT1, first cloned in 1994, has the broadest expression among MCTs 1 to 4 and is often highly expressed in cells with high oxidative activity, such as oxidative muscle fibers (12–14). MCT1 was also found in the mitochondria of cardiac and skeletal muscle (15). MCT4 expression is also correlated with high glycolytic activity of the skeletal muscle (13, 16). In addition, MCT2 has little expression in skeletal muscle (17), while MCT3 is restricted to the basal membrane of retinal pigment epithelium and choroid plexus epithelium (1).
Recently, human mutation studies and mouse models with disruption of genes involved in lactate transporters have aided in elucidating the physiological and pathophysiological functions of lactate transport in a tissue-specific manner, thereby highlighting MCT1 as an important player in numerous physiological processes and diseases. Inactivation of MCT1 caused by mutations leads to defective utilization of ketone bodies and consequently results in recurrent ketoacidosis in children (18). Homozygous MCT1 deletion (Slc16a1−/−) in mice led to embryonic lethality, while the haploinsufficient mice (Slc16a1+/−) were resistant to diet-induced obesity and associated metabolic perturbations (19). Studies with heterozygous Slc16a1+/− mice also indicated the role of MCT1 in the regulation of pH homeostasis and cellular energy homeostasis in skeletal muscles (20). Because of the limitation of whole-body deletion of Slc16a1, tissue-specific Slc16a1 knockout mouse models have been recently used to investigate the biological functions of MCT1 in various tissues/cells. Macrophage-specific deletion of Slc16a1 can prevent M2-like polarization of macrophages, leading to impairment of muscle reperfusion and regeneration from ischemia (21). Slc16a1 deletion in macrophages was also found to affect peripheral nerve regeneration in mice (22). Slc16a1 deficiency in adipocytes stimulated macrophage-mediated inflammation, consequently leading to high-fat diet (HFD)-induced insulin resistance in peripheral tissues (23). Hepatic deletion of Slc16a1 aggravated HFD-induced obesity in female mice but not in male mice (24). Deficiency of MCT1 in CD8+ T lymphocytes affects the proliferation and recruitment of the cells to the adipocyte tissues in obesity (25). However, deletion of MCT4 was reported to result in exercise intolerance, associated with structural degeneration of the neuromuscular junctions (25). In this study, we analyzed a mouse model with specific deletion of Slc16a1 in skeletal muscle. We discovered that MCT1-mediated lactate shuttle has an active role in promoting mitochondrial biogenesis and TCA flux, in addition to the known function of lactate as an energy fuel to feed the TCA cycle.
RESULTS
Deletion of Slc16a1 in skeletal muscle increases metabolic rate and improves glucose tolerance in mice
We first explored the capacity of skeletal muscle to metabolize glucose and lactate in vivo. Serum samples were collected from left ventricle and femoral vein of the mice and then analyzed by liquid chromatography–mass spectrometry (fig. S1A). Among the potential fuels that feed TCA cycle, glucose and lactate were the major fuels consumed by skeletal muscle (fig. S1B). Next, we intravenously infused U-13C-glucose or U-13C-lactate and measured their contribution to glycolytic and TCA metabolites (Fig. 1A). Serum and skeletal muscles were collected at pseudo-steady state (2.5 hours after infusion). In theory, intramuscle lactate can be taken in directly from circulation or generated from glucose via glycolysis inside the muscle cells. We found that the normalized 13C level of lactate is higher from 13C-lactate infusion than from 13C-glucose infusion in all the muscle groups tested, including quadriceps (QUA), gastrocnemius (GAS), tibialis anterior (TA), and soleus (SOL) (Fig. 1B). Consequently, skeletal muscles contained more 13C-labeled glycolytic product and TCA metabolites (pyruvate, succinate, and malate) from 13C-lactate than from 13C-glucose (Fig. 1B). We also investigated the direct contribution of these two circulatory nutrients to intramuscle lactate and malate via 13C labeling matrix deconvolution (Fig. 1C and fig. S1C). On the one hand, our analysis revealed that circulatory lactate contributed to ~50% of intramuscle lactate in QUA, TA, and GAS and ~40% of intramuscle lactate in SOL (Fig. 1D). On the other hand, circulatory glucose contributed to <5% of intramuscle lactate in QUA, TA, and GAS and ~20% of intramuscle lactate in SOL (Fig. 1D). Notably, intramuscle malate, a representative TCA metabolite, was exclusively from circulatory lactate in all the muscle groups (Fig. 1D). These results indicate that lactate is the preferred fuel for TCA cycle in skeletal muscle, partly consistent with a previous report (5).
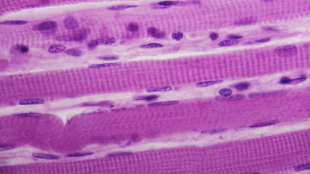
Lactate is transported from the circulation to the skeletal muscle via MCTs. Among the 16 MCTs in mammals, MCT1 (Slc16a1), MCT2 (Slc16a7), and MCT4 (Slc16a3) are involved in lactate transport in the skeletal muscle (1, 4). In mice, we found that MCT1 had the highest mRNA level among these three transporters in the skeletal muscle (fig. S1D). Among the four types of skeletal muscles with different oxidation capacity, MCT1 had the highest expression level in SOL, a muscle type with high oxidative capacity, followed by TA, GAS, and QUA, which are mainly fast muscle types with high glycolytic capacity (fig. S1E). Immunofluorescence staining of the skeletal muscle also revealed that MCT1 protein had higher distribution in SOL than in GAS and TA (fig. S1F). These observations are in line with previous findings demonstrating that the expression of MCT1 in individual muscle type is positively correlated with mitochondrial content and oxidation capacity (1, 4, 13). Also consistent with previous reports, MCT1 was expressed in the membranes of type I, type IIA, and type IIX myofibers while absent in type IIB myofibers that have the highest glycolytic activity among the four major muscle fiber types (Fig. 1E and fig. S1G). Conversely, MCT4 was present in type IIB and IIX fibers but barely detectable in type I and IIA fibers (fig. S1H), consistent with previous reports (13, 16).
As our analysis indicated that MCT1 is the major type of MCTs in the skeletal muscle, we focused our study on MCT1 using a skeletal muscle-specific knockout (mKO) mouse model (Fig. 1F). As expected, the mKO mice expressed little MCT1 in different types of skeletal muscle in comparison with the wild-type (WT) littermates, shown by quantitative real-time polymerase chain reaction (PCR) and immunofluorescence staining (Fig. 1G and fig. S2A). In addition, MCT1 protein level in the plasma membrane and mitochondria fractions was reduced in the skeletal muscle of mKO mice (fig. S2B). In GAS, expressions of both MCT2 and MCT4 were not altered by MCT1 deletion (fig. S2C). In SOL, expression of both MCT2 and MCT4 were reduced by MCT1 deletion (fig. S2C). We also analyzed the protein levels of lactate dehydrogenase A (LDHA), lactate dehydrogenase B (LDHB), and mitochondrial carrier protein 2 (MPC2) using mitochondria fraction from GAS muscle. While LDHA was decreased in mKO mice, LDHB and MPC2 were slightly increased in mKO mice (fig. S2D).
The body weight and lean mass were reduced in mKO mice under normal chow (Fig. 1, H and I). Consistently, we found that the weights of several muscle were reduced in mKO mice (Fig. 1J). Analysis with metabolic cage revealed increases in carbon dioxide production, oxygen consumption, daytime respiratory exchange ratio (RER), and night resting energy expenditure (REE) in mKO mice (Fig. 1K and fig. S2E). The increase in RER might reflect more energy substrate usage from carbohydrate than usage from lipid. To exclude the possibility that the loss of body weight was affected by appetite in mKO mice, we tracked food intake for seven consecutive days, and more food intake was increased in many time points in mKO mice (fig. S2F). In addition, mKO mice had improvement in glucose tolerance (Fig. 1L), while the fasting plasma insulin level and insulin tolerance were not altered (fig. S2, G and H). Collectively, these data indicate that deficiency of MCT1 in skeletal muscle reduces body weight while enhances metabolic rate and improves glucose tolerance in mice.
Deficiency of MCT1 in skeletal muscle elevates intramuscular lactate level, improves running endurance, and switches myofibers toward oxidative type
As MCT1 is a major transporter of lactate, we detected lactate level in the skeletal muscle. Both SOL and QUA had higher lactate level in mKO mice than the WT littermates (Fig. 2A). Moreover, blood lactate level was notably decreased in mKO mice in a treadmill running test (Fig. 2B). Notably, mKO mice had better performance in running endurance when subjected to treadmill for exhausted exercise (Fig. 2C). However, maximal gripping force which mainly tests for the strength of fast glycolytic muscles was reduced in the mKO mice (Fig. 2D).
We next used immunofluorescence staining to determine different types of muscle fibers. mKO mice had a notable increase in the proportion of fast-twitch oxidative IIA fibers, together with a decrease in the proportion of fast-twitch glycolytic IIB fibers in SOL, GAS, and TA (Fig. 2E). It is worth noting that a small percentage of IIB fibers existed in SOL in the WT mice, and these fibers were almost completely disappeared in mKO mice (Fig. 2E and fig. S3A). Highly oxidative type I fibers were almost absent in GAS of the WT mice but started to appear in mKO mice though (Fig. 2E and fig. S3B). In addition to the changes of myofiber composition, a cross-sectional area tended to be reduced in SOL of mKO mice (fig. S3C). Together, these data indicate that loss of MCT1 in skeletal muscle promotes switch of myofibers from glycolytic type to oxidative type. Collectively, these data reveal that loss of MCT1 in skeletal muscle elevates local lactate level, enhances running endurance, and promotes switch of muscle fibers toward oxidative type.
Loss of MCT1 increases glycolytic flux and TCA flux of glucose in the skeletal muscle
To explore the impact of MCT1 deficiency on metabolic flux in skeletal muscle, we used intravenous infusion of 13C-labeled glucose and 13C-labeled lactate to measure their contributions to glycolytic and TCA cycle in the skeletal muscle as described in Fig. 1A. Overall, the contribution of glucose to glycolysis and TCA was increased by MCT1 deletion (Fig. 3A and fig. S4A), while the contribution of lactate was reduced in mKO mice (Fig. 3D and fig. S4B). In particular, the normalized direct contributions of blood glucose to intramuscle lactate and malate were elevated in all the muscle groups tested in mKO mice (Fig. 3, B and C). In contrast, direct contributions of blood lactate to intramuscle lactate and malate were reduced when MCT1 was deleted (Fig. 3, E and F)….