Abstract
Macrophages have diverse functions in immunity as well as development and homeostasis. Here we identify a function for these cells in long distance communication during postembryonic tissue remodeling. Ablation of macrophages in zebrafish prevented melanophores from coalescing into adult pigment stripes. Melanophore organization depends on signals provided by cells of the yellow xanthophore lineage via airinemes, long filamentous projections with vesicles at their tips. We show that airineme extension from originating cells, and vesicle deposition on target cells, depend on interactions with macrophages. These findings identify a role for macrophages in relaying long range signals between nonimmune cells. It will be interesting to see if this signaling modality functions in the remodeling and homeostasis of other tissues during normal development and disease.
Macrophages are phagocytic cells with essential roles in immunity including recognition and disposal of infectious microbes, dying cells and debris. Yet, nonimmune activities have also been identified. Macrophages are now known to function during development and homeostasis, including blood vessel and mammary duct morphogenesis, pancreatic cell specification, hematopoietic stem cell maintenance, and lipid metabolism (1–4). To investigate potential roles for macrophages in postembryonic tissue remodeling, we examined the larval-to-adult transformation of zebrafish, a period of morphogenesis, patterning and growth, with similarities to human fetal and neonatal development (5, 6).
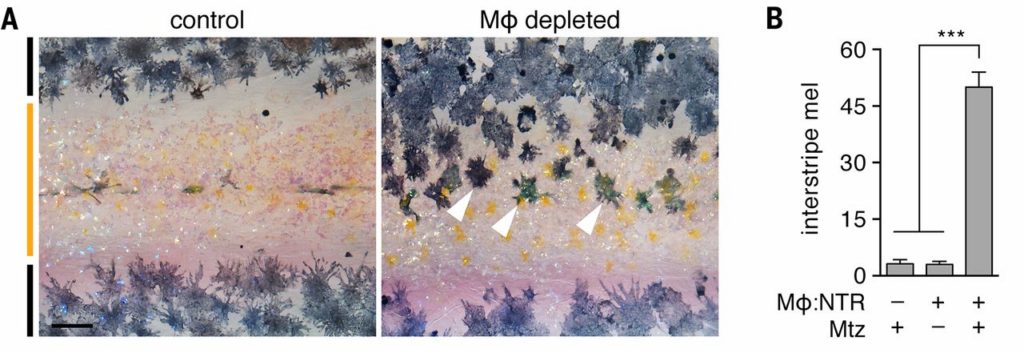
We depleted macrophage populations by expressing bacterial nitroreductase (NTR) in these cells. NTR kills cells by converting metronidazole (Mtz) to toxic metabolites (7, 8) (fig. S1). Fish treated with Mtz between mid-larval and juvenile stages had a severe defect in the adult pattern of neural crest derived pigment cells. Untreated juvenile zebrafish exhibit dark stripes of black melanophores dorsal and ventral to a light “interstripe” of yellow-orange xanthophores and iridescent iridophores. Yet, macrophage-depleted fish retained numerous melanophores in the interstripe (Fig. 1A and fig. S2). This pattern resembled a phenotype that results from a defect in long distance communication between melanophores and xanthophore precursors (9). During stripe development, progenitors of adult melanophores migrate to the skin and begin to differentiate widely over the flank (10). At this stage, precursors to adult xanthophores are already located in the prospective interstripe, where they differentiate as xanthophores, and also in prospective stripes, where they remain as unpigmented xanthoblasts (6) (Fig. 2A). Interactions between melanophores and cells of the xanthophore lineage are required for pattern formation and maintenance (11–14); e.g., xanthophores repel melanophores at short range (15, 16). By contrast, xanthoblasts extend thin projections, “airinemes,” that contact dispersed melanophores and melanoblasts (9). Airinemes arise from surface blebs, can reach up to several cell diameters (>150 μm), and have large (~1 μm) membrane-bound vesicles at their tips that harbor the Notch pathway ligand DeltaC and potentially other factors. When xanthoblasts are ablated, or when airineme production or Delta–Notch signaling are inhibited, melanophores fail to organize and many persist in the interstripe (9). Given the similarity of this phenotype to that of macrophage-depletion, we speculated that macrophages contribute to airineme-dependent signaling.